A Phenotype-Driven Approach to Generate Mouse Models with Pathogenic mtDNA Mutations Causing Mitochondrial Disease
- PMID: 27626666
- PMCID: PMC5039181
- DOI: 10.1016/j.celrep.2016.08.037
A Phenotype-Driven Approach to Generate Mouse Models with Pathogenic mtDNA Mutations Causing Mitochondrial Disease
Abstract
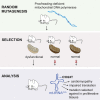
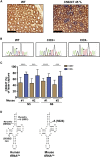
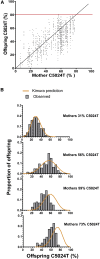
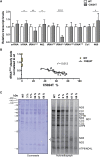
Mutations of mtDNA are an important cause of human disease, but few animal models exist. Because mammalian mitochondria cannot be transfected, the development of mice with pathogenic mtDNA mutations has been challenging, and the main strategy has therefore been to introduce mutations found in cell lines into mouse embryos. Here, we describe a phenotype-driven strategy that is based on detecting clonal expansion of pathogenic mtDNA mutations in colonic crypts of founder mice derived from heterozygous mtDNA mutator mice. As proof of concept, we report the generation of a mouse line transmitting a heteroplasmic pathogenic mutation in the alanine tRNA gene of mtDNA displaying typical characteristics of classic mitochondrial disease. In summary, we describe a straightforward and technically simple strategy based on mouse breeding and histology to generate animal models of mtDNA-mutation disease, which will be of great importance for studies of disease pathophysiology and preclinical treatment trials.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ciafaloni E., Ricci E., Servidei S., Shanske S., Silvestri G., Manfredi G., Schon E.A., DiMauro S. Widespread tissue distribution of a tRNALeu(UUR) mutation in the mitochondrial DNA of a patient with MELAS syndrome. Neurology. 1991;41:1663–1664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

