Sequential and Simultaneous Immunization of Rabbits with HIV-1 Envelope Glycoprotein SOSIP.664 Trimers from Clades A, B and C
- PMID: 27627672
- PMCID: PMC5023125
- DOI: 10.1371/journal.ppat.1005864
Sequential and Simultaneous Immunization of Rabbits with HIV-1 Envelope Glycoprotein SOSIP.664 Trimers from Clades A, B and C
Abstract
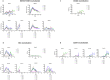
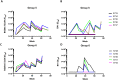
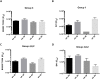
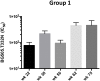
We have investigated the immunogenicity in rabbits of native-like, soluble, recombinant SOSIP.664 trimers based on the env genes of four isolates of human immunodeficiency virus type 1 (HIV-1); specifically BG505 (clade A), B41 (clade B), CZA97 (clade C) and DU422 (clade C). The various trimers were delivered either simultaneously (as a mixture of clade A + B trimers) or sequentially over a 73-week period. Autologous, Tier-2 neutralizing antibody (NAb) responses were generated to the clade A and clade B trimers in the bivalent mixture. When delivered as boosting immunogens to rabbits immunized with the clade A and/or clade B trimers, the clade C trimers also generated autologous Tier-2 NAb responses, the CZA97 trimers doing so more strongly and consistently than the DU422 trimers. The clade C trimers also cross-boosted the pre-existing NAb responses to clade A and B trimers. We observed heterologous Tier-2 NAb responses albeit inconsistently, and with limited overall breath. However, cross-neutralization of the clade A BG505.T332N virus was consistently observed in rabbits immunized only with clade B trimers and then boosted with clade C trimers. The autologous NAbs induced by the BG505, B41 and CZA97 trimers predominantly recognized specific holes in the glycan shields of the cognate virus. The shared location of some of these holes may account for the observed cross-boosting effects and the heterologous neutralization of the BG505.T332N virus. These findings will guide the design of further experiments to determine whether and how multiple Env trimers can together induce more broadly neutralizing antibody responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

