Slow gamma rhythms in CA3 are entrained by slow gamma activity in the dentate gyrus
- PMID: 27628206
- PMCID: PMC5133296
- DOI: 10.1152/jn.00499.2016
Slow gamma rhythms in CA3 are entrained by slow gamma activity in the dentate gyrus
Abstract
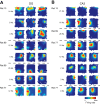
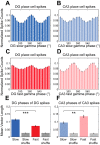
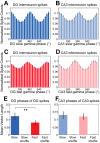
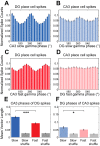
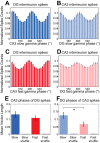
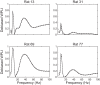
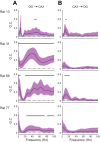
In hippocampal area CA1, slow (∼25-55 Hz) and fast (∼60-100 Hz) gamma rhythms are coupled with different CA1 afferents. CA1 slow gamma is coupled to inputs from CA3, and CA1 fast gamma is coupled to inputs from the medial entorhinal cortex (Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Nature 462: 353-357, 2009). CA3 gives rise to highly divergent associational projections, and it is possible that reverberating activity in these connections generates slow gamma rhythms in the hippocampus. However, hippocampal gamma is maximal upstream of CA3, in the dentate gyrus (DG) region (Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. J Neurosci 15: 47-60, 1995). Thus it is possible that slow gamma in CA3 is driven by inputs from DG, yet few studies have examined slow and fast gamma rhythms in DG recordings. Here we investigated slow and fast gamma rhythms in paired recordings from DG and CA3 in freely moving rats to determine whether slow and fast gamma rhythms in CA3 are entrained by DG. We found that slow gamma rhythms, as opposed to fast gamma rhythms, were particularly prominent in DG. We investigated directional causal influences between DG and CA3 by Granger causality analysis and found that DG slow gamma influences CA3 slow gamma. Moreover, DG place cell spikes were strongly phase-locked to CA3 slow gamma rhythms, suggesting that DG excitatory projections to CA3 may underlie this directional influence. These results indicate that slow gamma rhythms do not originate in CA3 but rather slow gamma activity upstream in DG entrains slow gamma rhythms in CA3.
Keywords: CA3; dentate gyrus; gamma rhythms.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Barnett L, Seth AK. The MVGC multivariate Granger causality toolbox: a new approach to Granger-causal inference. J Neurosci Methods 223: 50–68, 2014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

