Fine-tuning of amino sugar homeostasis by EIIA(Ntr) in Salmonella Typhimurium
- PMID: 27628932
- PMCID: PMC5024086
- DOI: 10.1038/srep33055
Fine-tuning of amino sugar homeostasis by EIIA(Ntr) in Salmonella Typhimurium
Abstract
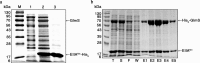
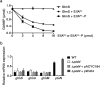
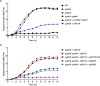
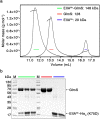
The nitrogen-metabolic phosphotransferase system, PTS(Ntr), consists of the enzymes I(Ntr), NPr and IIA(Ntr) that are encoded by ptsP, ptsO, and ptsN, respectively. Due to the proximity of ptsO and ptsN to rpoN, the PTS(Ntr) system has been postulated to be closely related with nitrogen metabolism. To define the correlation between PTS(Ntr) and nitrogen metabolism, we performed ligand fishing with EIIA(Ntr) as a bait and revealed that D-glucosamine-6-phosphate synthase (GlmS) directly interacted with EIIA(Ntr). GlmS, which converts D-fructose-6-phosphate (Fru6P) into D-glucosamine-6-phosphate (GlcN6P), is a key enzyme producing amino sugars through glutamine hydrolysis. Amino sugar is an essential structural building block for bacterial peptidoglycan and LPS. We further verified that EIIA(Ntr) inhibited GlmS activity by direct interaction in a phosphorylation-state-dependent manner. EIIA(Ntr) was dephosphorylated in response to excessive nitrogen sources and was rapidly degraded by Lon protease upon amino sugar depletion. The regulation of GlmS activity by EIIA(Ntr) and the modulation of glmS translation by RapZ suggest that the genes comprising the rpoN operon play a key role in maintaining amino sugar homeostasis in response to nitrogen availability and the amino sugar concentration in the bacterial cytoplasm.
Figures



References
-
- Görke B. & Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6, 613–624 (2008). - PubMed
-
- Lengeler J. W. & Jahreis K. Bacterial PEP-dependent carbohydrate: phosphotransferase systems couple sensing and global control mechanisms. Contrib. Microbiol. 16, 65–87 (2009). - PubMed
-
- Escalante A., Cervantes A. S., Gosset G. & Bolivar F. Current knowledge of the Escherichia coli phosphoenolpyruvate-carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation. Appl. Microbiol. Biotechnol. 94, 1483–1494 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

