Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study
- PMID: 27629100
- PMCID: PMC5531346
- DOI: 10.1177/0271678X16669366
Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study
Abstract
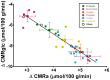
Ketones (principally β-hydroxybutyrate and acetoacetate (AcAc)) are an important alternative fuel to glucose for the human brain, but their utilisation by the brain remains poorly understood. Our objective was to use positron emission tomography (PET) to assess the impact of diet-induced moderate ketosis on cerebral metabolic rate of acetoacetate (CMRa) and glucose (CMRglc) in healthy adults. Ten participants (35 ± 15 y) received a very high fat ketogenic diet (KD) (4.5:1; lipid:protein plus carbohydrates) for four days. CMRa and CMRglc were quantified by PET before and after the KD with the tracers, 11C-AcAc and 18F-fluorodeoxyglucose (18F-FDG), respectively. During the KD, plasma ketones increased 8-fold ( p = 0.005) while plasma glucose decreased by 24% ( p = 0.005). CMRa increased 6-fold ( p = 0.005), whereas CMRglc decreased by 20% ( p = 0.014) on the KD. Plasma ketones were positively correlated with CMRa (r = 0.93; p < 0.0001). After four days on the KD, CMRa represented 17% of whole brain energy requirements in healthy adults with a 2-fold difference across brain regions (12-24%). The CMR of ketones (AcAc and β-hydroxybutyrate combined) while on the KD was estimated to represent about 33% of brain energy requirements or approximately double the CMRa. Whether increased ketone availability raises CMR of ketones to the same extent in older people as observed here or in conditions in which chronic brain glucose hypometabolism is present remains to be determined.
Keywords: Metabolism; acetoacetate; glucose; ketogenic diet; ketone; neuroimaging.
Figures




References
-
- Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 2008; 7: 500–506. - PubMed
-
- Phelps ME, Huang SC, Hoffman EJ, et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 1979; 6: 371–388. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

