Amyloid structure exhibits polymorphism on multiple length scales in human brain tissue
- PMID: 27629394
- PMCID: PMC5024092
- DOI: 10.1038/srep33079
Amyloid structure exhibits polymorphism on multiple length scales in human brain tissue
Abstract
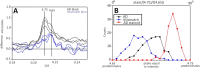
Aggregation of Aβ amyloid fibrils into plaques in the brain is a universal hallmark of Alzheimer's Disease (AD), but whether plaques in different individuals are equivalent is unknown. One possibility is that amyloid fibrils exhibit different structures and different structures may contribute differentially to disease, either within an individual brain or between individuals. However, the occurrence and distribution of structural polymorphisms of amyloid in human brain is poorly documented. Here we use X-ray microdiffraction of histological sections of human tissue to map the abundance, orientation and structural heterogeneities of amyloid. Our observations indicate that (i) tissue derived from subjects with different clinical histories may contain different ensembles of fibrillar structures; (ii) plaques harboring distinct amyloid structures can coexist within a single tissue section and (iii) within individual plaques there is a gradient of fibrillar structure from core to margins. These observations have immediate implications for existing theories on the inception and progression of AD.
Figures






References
-
- Hardy J. & Selkoe D. J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
-
- Selkoe D. J. Resolving controversies on the path to Alzheimer’s therapeutics. Nature medicine, 17, 1060–1065 (2011). - PubMed
-
- Benilova I., Karran E. & De S. B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 15, 349–357 (2012). - PubMed
-
- Price J. L. & Morris J. C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 45, 358–368 (1999). - PubMed
-
- Dickson D. W. et al.. Identification of normal and pathological aging in prospectively studied non-demented elderly humans. Neurobiol. Aging 13, 179–189 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

