The Role of Striatal Feedforward Inhibition in the Maintenance of Absence Seizures
- PMID: 27629713
- PMCID: PMC6601939
- DOI: 10.1523/JNEUROSCI.0208-16.2016
The Role of Striatal Feedforward Inhibition in the Maintenance of Absence Seizures
Abstract
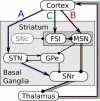
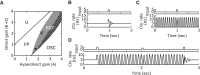
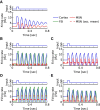
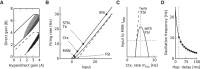
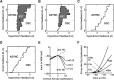
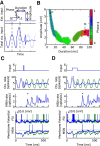
Absence seizures are characterized by brief interruptions of conscious experience accompanied by oscillations of activity synchronized across many brain areas. Although the dynamics of the thalamocortical circuits are traditionally thought to underlie absence seizures, converging experimental evidence supports the key involvement of the basal ganglia (BG). In this theoretical work, we argue that the BG are essential for the maintenance of absence seizures. To this end, we combine analytical calculations with numerical simulations to investigate a computational model of the BG-thalamo-cortical network. We demonstrate that abnormally strong striatal feedforward inhibition can promote synchronous oscillatory activity that persists in the network over several tens of seconds as observed during seizures. We show that these maintained oscillations result from an interplay between the negative feedback through the cortico-subthalamo-nigral pathway and the striatal feedforward inhibition. The negative feedback promotes epileptic oscillations whereas the striatal feedforward inhibition suppresses the positive feedback provided by the cortico-striato-nigral pathway. Our theory is consistent with experimental evidence regarding the influence of BG on seizures (e.g., with the fact that a pharmacological blockade of the subthalamo-nigral pathway suppresses seizures). It also accounts for the observed strong suppression of the striatal output during seizures. Our theory predicts that well-timed transient excitatory inputs to the cortex advance the termination of absence seizures. In contrast with the thalamocortical theory, it also predicts that reducing the synaptic transmission along the cortico-subthalamo-nigral pathway while keeping constant the average firing rate of substantia nigra pars reticulata reduces the incidence of seizures.
Significance statement: Absence seizures are characterized by brief interruptions of consciousness accompanied by abnormal brain oscillations persisting tens of seconds. Thalamocortical circuits are traditionally thought to underlie absence seizures. However, recent experiments have highlighted the key role of the basal ganglia (BG). This work argues for a novel theory according to which the BG drive the oscillatory patterns of activity occurring during the seizures. It demonstrates that abnormally strong striatal feedforward inhibition promotes synchronous oscillatory activity in the BG-thalamo-cortical network and relate this property to the observed strong suppression of the striatal output during seizures. The theory is compatible with virtually all known experimental results, and it predicts that well-timed transient excitatory inputs to the cortex advance the termination of absence seizures.
Keywords: bistability; computational model; dynamics; fast spiking interneuron; oscillations; spike-and-wave discharges.
Copyright © 2016 the authors 0270-6474/16/369618-15$15.00/0.
Figures


References
-
- Arakaki T, Leblois A, Charpier S, Hansel D. Persistence and termination of absence epileptic seizures: the role of striatal feedforward inhibition. Neuroscience 2013 Abstracts, San Diego, Program 53.20. Society for Neuroscience. 2013 - PubMed
-
- Avanzini G, de Curtis M, Marescaux C, Panzica F, Spreafico R, Vergnes M. Role of the thalamic reticular nucleus in the generation of rhythmic thalamo-cortical activities subserving spike and waves. J Neural Transm Suppl. 1992;35:85–95. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
