Intratumoral Photodynamic Therapy With Newly Synthesized Pheophorbide a in Murine Oral Cancer
- PMID: 27629775
- PMCID: PMC7841246
- DOI: 10.3727/096504016X14732527645922
Intratumoral Photodynamic Therapy With Newly Synthesized Pheophorbide a in Murine Oral Cancer
Abstract
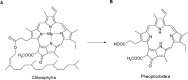
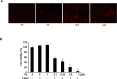
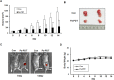
Photodynamic therapy (PDT) is a therapeutic alternative for malignant tumors that uses a photosensitizer. Our group recently synthesized photosensitizer pheophorbide a (Pa) from chlorophyll-a. The present study investigated the therapeutic effect of PDT using intratumoral administration of the synthetic photosensitizer Pa in an in vivo murine oral squamous cell carcinoma (OSCC) animal model. Pa accumulation was measured using the fluorescence spectrum and imaging in living C3H mice. Intratumoral treatment of Pa-PDT (IT Pa-PDT) significantly inhibited the growth of transplanted OSCC cells. Histopathological examination of tumor tissues showed that PCNA expression was significantly decreased, while TUNEL-stained cells were markedly increased in the IT Pa-PDT group compared to controls. IT Pa-PDT-induced apoptosis was confirmed by immunoblot. Reduction of Bcl-2 and cleavage of caspase 3 and PARP were observed in IT Pa-PDT. These data demonstrate that IT Pa-PDT inhibited tumor cell proliferation and induced apoptosis, which is correlated with the anticancer activity of IT Pa-PDT. These potent antitumor activities of IT Pa-PDT were observed in both the immunohistochemistry and Western blot experiments. Our findings suggest the intratumoral therapeutic potential of Pa-PDT on OSCC. Additionally, demonstrated detection of Pa using a fluorescence spectroscopy system or molecular imaging system provides a means for simultaneous diagnosis and treatment of OSCC.
Figures



References
-
- Marmur ES, Schmults CD, Goldberg J. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2004;30:264–71. - PubMed
-
- Weishaupt KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976;36:2326–9. - PubMed
-
- Moan J, Berg K. Photochemotherapy of cancer: Experimental research. Photochem Photobiol. 1992;55:931–48. - PubMed
-
- Karakullukcu B, Nyst HJ, van Veen RL, Hoebers FJ, Hamming-Vrieze O, Witjes MJ, de Visscher SA, Burlage FR, Levendag PC, Sterenborg HJ, Tan IB. mTHPC mediated interstitial photodynamic therapy of recurrent nonmetastatic base of tongue cancers: Development of a new method. Head Neck 2012;34:1597–1606. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
