Lone Atrial Fibrillation Is Associated With Impaired Left Ventricular Energetics That Persists Despite Successful Catheter Ablation
- PMID: 27630135
- PMCID: PMC5054971
- DOI: 10.1161/CIRCULATIONAHA.116.022931
Lone Atrial Fibrillation Is Associated With Impaired Left Ventricular Energetics That Persists Despite Successful Catheter Ablation
Abstract
Background: Lone atrial fibrillation (AF) may reflect a subclinical cardiomyopathy that persists after sinus rhythm (SR) restoration, providing a substrate for AF recurrence. To test this hypothesis, we investigated the effect of restoring SR by catheter ablation on left ventricular (LV) function and energetics in patients with AF but no significant comorbidities.
Methods: Fifty-three patients with symptomatic paroxysmal or persistent AF and without significant valvular disease, uncontrolled hypertension, coronary artery disease, uncontrolled thyroid disease, systemic inflammatory disease, diabetes mellitus, or obstructive sleep apnea (ie, lone AF) undergoing ablation and 25 matched control subjects in SR were investigated. Magnetic resonance imaging quantified LV ejection fraction (LVEF), peak systolic circumferential strain (PSCS), and left atrial volumes and function, whereas phosphorus-31 magnetic resonance spectroscopy evaluated ventricular energetics (ratio of phosphocreatine to ATP). AF burden was determined before and after ablation by 7-day Holter monitoring; intermittent ECG event monitoring was also undertaken after ablation to investigate for asymptomatic AF recurrence.
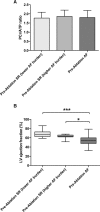
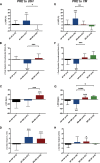
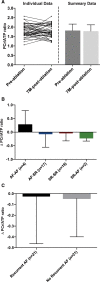
Results: Before ablation, both LV function and energetics were significantly impaired in patients compared with control subjects (LVEF, 61% [interquartile range (IQR), 52%-65%] versus 71% [IQR, 69%-73%], P<0.001; PSCS, -15% [IQR, -11 to -18%] versus -18% [IQR, -17% to -19%], P=0.002; ratio of phosphocreatine to ATP, 1.81±0.35 versus 2.05±0.29, P=0.004). As expected, patients also had dilated and impaired left atria compared with control subjects (all P<0.001). Early after ablation (1-4 days), LVEF and PSCS improved in patients recovering SR from AF (LVEF, 7.0±10%, P=0.005; PSCS, -3.5±4.3%, P=0.001) but were unchanged in those in SR during both assessments (both P=NS). At 6 to 9 months after ablation, AF burden reduced significantly (from 54% [IQR, 1.5%-100%] to 0% [IQR 0%-0.1%]; P<0.001). However, LVEF and PSCS did not improve further (both P=NS) and remained impaired compared with control subjects (P<0.001 and P=0.003, respectively). Similarly, there was no significant improvement in atrial function from before ablation (P=NS), and this remained lower than in control subjects (P<0.001). The ratio of phosphocreatine to ATP was unaffected by heart rhythm during assessment and AF burden before ablation (both P=NS). It was unchanged after ablation (P=0.57), remaining lower than in control subjects regardless of both recovery of SR and freedom from recurrent AF (P=0.006 and P=0.002, respectively).
Conclusions: Patients with lone AF have impaired myocardial energetics and subtle LV dysfunction, which do not normalize after ablation. These findings suggest that AF may be the consequence (rather than the cause) of an occult cardiomyopathy, which persists despite a significant reduction in AF burden after ablation.
Keywords: arrhythmias, cardiac; atrial fibrillation; catheter ablation; magnetic resonance imaging; magnetic resonance spectroscopy; ventricular dysfunction, left.
© 2016 The Authors.
Figures


Comment in
-
Mental Exit Block: Escaping the Pulmonary Veins in Search of New Approaches to Atrial Fibrillation Management.Circulation. 2016 Oct 11;134(15):1082-1084. doi: 10.1161/CIRCULATIONAHA.116.024636. Epub 2016 Sep 14. Circulation. 2016. PMID: 27630134 No abstract available.
References
-
- Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB. Stroke severity in atrial fibrillation: the Framingham study. Stroke. 1996;27:1760–1764. - PubMed
-
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. - PubMed
-
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. - PMC - PubMed
-
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

