Lipoxin A4, a 5-lipoxygenase pathway metabolite, modulates immune response during acute respiratory tularemia
- PMID: 27630217
- PMCID: PMC5235906
- DOI: 10.1189/jlb.4A0815-365RR
Lipoxin A4, a 5-lipoxygenase pathway metabolite, modulates immune response during acute respiratory tularemia
Abstract
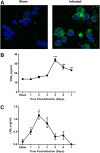
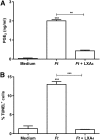
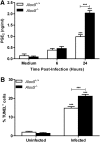
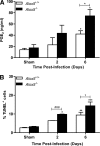
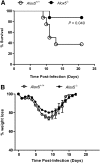
Respiratory infection with Francisella tularensis (Ft) is characterized by a muted, acute host response, followed by sepsis-like syndrome that results in death. Infection with Ft establishes a principally anti-inflammatory environment that subverts host-cell death programs to facilitate pathogen replication. Although the role of cytokines has been explored extensively, the role of eicosanoids in tularemia pathogenesis is not fully understood. Given that lipoxin A4 (LXA4) has anti-inflammatory properties, we investigated whether this lipid mediator affects host responses manifested early during infection. The addition of exogenous LXA4 inhibits PGE2 release by Ft-infected murine monocytes in vitro and diminishes apoptotic cell death. Tularemia pathogenesis was characterized in 5‑lipoxygenase-deficient (Alox5-/-) mice that are incapable of generating LXA4 Increased release of proinflammatory cytokines and chemokines, as well as increased apoptosis, was observed in Alox5-/- mice as compared with their wild-type counterparts. Alox5-/- mice also exhibited elevated recruitment of neutrophils during the early phase of infection and increased resistance to lethal challenge. Conversely, administration of exogenous LXA4 to Alox5-/- mice made them more susceptible to infection thus mimicking wild-type animals. Taken together, our results suggest that 5-LO activity is a critical regulator of immunopathology observed during the acute phase of respiratory tularemia, regulating bacterial burden and neutrophil recruitment and production of proinflammatory modulators and increasing morbidity and mortality. These studies identify a detrimental role for the 5-LO-derived lipid mediator LXA4 in Ft-induced immunopathology. Targeting this pathway may have therapeutic benefit as an adjunct to treatment with antibiotics and conventional antimicrobial peptides, which often have limited efficacy against intracellular bacteria.
Keywords: anti-inflammatory; cytokines; inflammation; lipid mediators.
© Society for Leukocyte Biology.
Figures
Similar articles
-
Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor alpha-mediated CINC-1 release in rat liver cells: novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4.FASEB J. 2002 Dec;16(14):1937-9. doi: 10.1096/fj.02-0224fje. Epub 2002 Oct 4. FASEB J. 2002. PMID: 12368230
-
Lipoxins exert antiangiogenic and anti-inflammatory effects on Kaposi's sarcoma cells.Transl Res. 2015 Aug;166(2):111-33. doi: 10.1016/j.trsl.2015.02.009. Epub 2015 Mar 6. Transl Res. 2015. PMID: 25814167
-
5-Lipoxygenase negatively regulates Th1 response during Brucella abortus infection in mice.Infect Immun. 2015 Mar;83(3):1210-6. doi: 10.1128/IAI.02592-14. Epub 2015 Jan 12. Infect Immun. 2015. PMID: 25583526 Free PMC article.
-
Dying to live: how the death modality of the infected macrophage affects immunity to tuberculosis.Adv Exp Med Biol. 2013;783:103-20. doi: 10.1007/978-1-4614-6111-1_6. Adv Exp Med Biol. 2013. PMID: 23468106 Free PMC article. Review.
-
Neutrophils: potential therapeutic targets in tularemia?Front Cell Infect Microbiol. 2013 Dec 27;3:109. doi: 10.3389/fcimb.2013.00109. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24409419 Free PMC article. Review.
Cited by
-
Host-based lipid inflammation drives pathogenesis in Francisella infection.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):12596-12601. doi: 10.1073/pnas.1712887114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109289 Free PMC article.
-
Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review.J Adv Res. 2018 Jan 3;11:57-66. doi: 10.1016/j.jare.2018.01.001. eCollection 2018 May. J Adv Res. 2018. PMID: 30034876 Free PMC article. Review.
-
Necroptotic debris including damaged mitochondria elicits sepsis-like syndrome during late-phase tularemia.Cell Death Discov. 2017 Sep 25;3:17056. doi: 10.1038/cddiscovery.2017.56. eCollection 2017. Cell Death Discov. 2017. PMID: 28955505 Free PMC article.
-
Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction.Haematologica. 2020 Aug;105(8):2056-2070. doi: 10.3324/haematol.2019.219519. Epub 2019 Nov 28. Haematologica. 2020. PMID: 31780628 Free PMC article.
References
-
- Periasamy S., Singh A., Sahay B., Rahman T., Feustel P. J., Pham G. H., Gosselin E. J., Sellati T. J. (2011) Development of tolerogenic dendritic cells and regulatory T cells favors exponential bacterial growth and survival during early respiratory tularemia. J. Leukoc. Biol. 90, 493–507. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

