Modeling of Cerebral Oxygen Transport Based on In vivo Microscopic Imaging of Microvascular Network Structure, Blood Flow, and Oxygenation
- PMID: 27630556
- PMCID: PMC5006088
- DOI: 10.3389/fncom.2016.00082
Modeling of Cerebral Oxygen Transport Based on In vivo Microscopic Imaging of Microvascular Network Structure, Blood Flow, and Oxygenation
Abstract
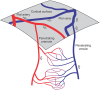
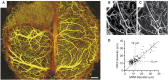
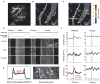
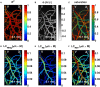
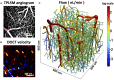
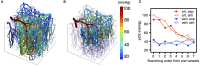
Oxygen is delivered to brain tissue by a dense network of microvessels, which actively control cerebral blood flow (CBF) through vasodilation and contraction in response to changing levels of neural activity. Understanding these network-level processes is immediately relevant for (1) interpretation of functional Magnetic Resonance Imaging (fMRI) signals, and (2) investigation of neurological diseases in which a deterioration of neurovascular and neuro-metabolic physiology contributes to motor and cognitive decline. Experimental data on the structure, flow and oxygen levels of microvascular networks are needed, together with theoretical methods to integrate this information and predict physiologically relevant properties that are not directly measurable. Recent progress in optical imaging technologies for high-resolution in vivo measurement of the cerebral microvascular architecture, blood flow, and oxygenation enables construction of detailed computational models of cerebral hemodynamics and oxygen transport based on realistic three-dimensional microvascular networks. In this article, we review state-of-the-art optical microscopy technologies for quantitative in vivo imaging of cerebral microvascular structure, blood flow and oxygenation, and theoretical methods that utilize such data to generate spatially resolved models for blood flow and oxygen transport. These "bottom-up" models are essential for the understanding of the processes governing brain oxygenation in normal and disease states and for eventual translation of the lessons learned from animal studies to humans.
Keywords: brain imaging methods; cerebral blood flow (CBF); cerebral blood flow measurement; cerebrovascular circulation; modeling and simulations.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

