Novel Insights Into the Immunoregulatory Function and Localization of Dendritic Cells
- PMID: 27631349
- PMCID: PMC5067968
- DOI: 10.1097/ICO.0000000000001005
Novel Insights Into the Immunoregulatory Function and Localization of Dendritic Cells
Abstract
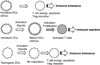
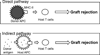
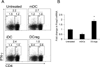
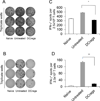
Dendritic cells (DCs) are antigen-presenting cells that normally play a critical role in stimulating T-cell-dependent immune responses. However, tolerogenic DCs (CD11cMHC-IICD80CD86) induce immune tolerance by stimulating regulatory T cells (Tregs: CD4CD25Foxp3). Although tolerogenic DCs are used to treat autoimmune diseases and to prevent transplantation rejection, the mechanisms by which they regulate alloimmunity are poorly understood. Here, we review our previous studies aiming to elucidate the mechanisms involved in immune rejection of corneal allografts using a corneal transplant model. We found that donor-derived tolerogenic DCs significantly prolonged corneal allograft survival by suppressing indirect allosensitization. We also reported the precise distribution of intraepithelial corneal DCs, termed Langerhans cells (LCs: CD11cLangerinMHC-II) in the cornea, which we maintain play a critical role in regulating corneal immunity. By confocal microscopy, we constructed 3-dimensional images of corneal LCs, which demonstrated that their cell bodies are present in the basal cell layer of the corneal epithelium. Furthermore, LC dendrites extend toward the ocular surface, but do not connect to epithelial tight junctions, indicating that they cannot directly interact with ocular surface antigens. We confirm the potential of DC therapy for corneal graft rejection and report the function of intraepithelial DCs (LCs) in the normal cornea.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Bol KF, Schreibelt G, Gerritsen WR, et al. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin Cancer Res. 2016;22(8):1897–1906. - PubMed
-
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. - PubMed
-
- Tan JK, O'Neill HC. Maturation requirements for dendritic cells in T cell stimulation leading to tolerance versus immunity. J Leukoc Biol. 2005;78(2):319–324. - PubMed
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

