Radicals: Reactive Intermediates with Translational Potential
- PMID: 27631602
- PMCID: PMC5054485
- DOI: 10.1021/jacs.6b08856
Radicals: Reactive Intermediates with Translational Potential
Abstract
This Perspective illustrates the defining characteristics of free radical chemistry, beginning with its rich and storied history. Studies from our laboratory are discussed along with recent developments emanating from others in this burgeoning area. The practicality and chemoselectivity of radical reactions enable rapid access to molecules of relevance to drug discovery, agrochemistry, material science, and other disciplines. Thus, these reactive intermediates possess inherent translational potential, as they can be widely used to expedite scientific endeavors for the betterment of humankind.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
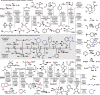
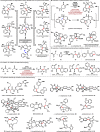
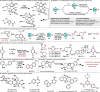

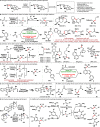

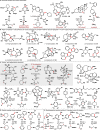
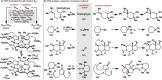
References
-
- Carey F. A.; Giuliano R. M.. Organic Chemistry, 10th ed.; McGraw-Hill Education: New York, 2016.
- Wade L. G.Organic Chemistry, 8th ed.; Pearson: New York, 2011.
- McMurry J.Organic Chemistry, 9th ed.; Cengage Learning: Boston, 2015.
- Clayden J.; Greeves N.; Warren S.. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, 2012.
-
-
For selected general reviews of radical chemistry, see:
- Kochi J. K., Ed. Free Radicals, Vol. 1: Dynamics of Elementary Processes; Wiley-Interscience: New York, 1973.
- Curran D. P. Synthesis 1988, 6, 417. 10.1055/s-1988-27600. - DOI
- Curran D. P. Synthesis 1988, 7, 489. 10.1055/s-1988-27620. - DOI
- Jasperse C. P.; Curran D. P.; Fevig T. L. Chem. Rev. 1991, 91, 1237. 10.1021/cr00006a006. - DOI
- Radicals in Organic Synthesis, 1st ed.; Renaud P., Sibi M., Eds.; Wiley-VCH: Weinheim, 2001.
- Togo H.Advanced Free Radical Reactions for Organic Synthesis; Elsevier: Amsterdam, 2003.
- Zard S. Z.Radical Reactions in Organic Synthesis; Oxford University Press: Oxford, 2003.
-
-
- Kolbe H. Ann. Chem. Pharm. 1848, 64, 339. 10.1002/jlac.18480640346. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

