Discovery of a Quinoline-4-carboxamide Derivative with a Novel Mechanism of Action, Multistage Antimalarial Activity, and Potent in Vivo Efficacy
- PMID: 27631715
- PMCID: PMC5108032
- DOI: 10.1021/acs.jmedchem.6b00723
Discovery of a Quinoline-4-carboxamide Derivative with a Novel Mechanism of Action, Multistage Antimalarial Activity, and Potent in Vivo Efficacy
Abstract
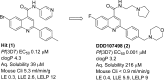
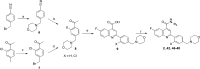
The antiplasmodial activity, DMPK properties, and efficacy of a series of quinoline-4-carboxamides are described. This series was identified from a phenotypic screen against the blood stage of Plasmodium falciparum (3D7) and displayed moderate potency but with suboptimal physicochemical properties and poor microsomal stability. The screening hit (1, EC50 = 120 nM) was optimized to lead molecules with low nanomolar in vitro potency. Improvement of the pharmacokinetic profile led to several compounds showing excellent oral efficacy in the P. berghei malaria mouse model with ED90 values below 1 mg/kg when dosed orally for 4 days. The favorable potency, selectivity, DMPK properties, and efficacy coupled with a novel mechanism of action, inhibition of translation elongation factor 2 (PfEF2), led to progression of 2 (DDD107498) to preclinical development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
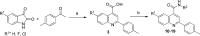
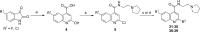

References
-
- WHO. World Malaria Report 2015; World Health Organization, 2015; 280 pp, http://www.who.int/malaria/publications/world-malaria-report-2015/report....
-
- Bhatt S.; Weiss D. J.; Cameron E.; Bisanzio D.; Mappin B.; Dalrymple U.; Battle K. E.; Moyes C. L.; Henry A.; Eckhoff P. A.; Wenger E. A.; Briet O.; Penny M. A.; Smith T. A.; Bennett A.; Yukich J.; Eisele T. P.; Griffin J. T.; Fergus C. A.; Lynch M.; Lindgren F.; Cohen J. M.; Murray C. L. J.; Smith D. L.; Hay S. I.; Cibulskis R. E.; Gething P. W. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. 10.1038/nature15535. - DOI - PMC - PubMed
-
- Tun K. M.; Imwong M.; Lwin K. M.; Win A. A.; Hlaing T. M.; Hlaing T.; Lin K.; Kyaw M. P.; Plewes K.; Faiz M. A.; Dhorda M.; Cheah P. Y.; Pukrittayakamee S.; Ashley E. A.; Anderson T. J.; Nair S.; McDew-White M.; Flegg J. A.; Grist E. P.; Guerin P.; Maude R. J.; Smithuis F.; Dondorp A. M.; Day N. P.; Nosten F.; White N. J.; Woodrow C. J. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect. Dis. 2015, 15, 415–421. 10.1016/S1473-3099(15)70032-0. - DOI - PMC - PubMed
- Phyo A. P.; Nkhoma S.; Stepniewska K.; Ashley E. A.; Nair S.; McGready R.; ler Moo C.; Al-Saai S.; Dondorp A. M.; Lwin K. M.; Singhasivanon P.; Day N. P.; White N. J.; Anderson T. J.; Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 2012, 379, 1960–1966. 10.1016/S0140-6736(12)60484-X. - DOI - PMC - PubMed
- Takala-Harrison S.; Jacob C. G.; Arze C.; Cummings M. P.; Silva J. C.; Dondorp A. M.; Fukuda M. M.; Hien T. T.; Mayxay M.; Noedl H.; Nosten F.; Kyaw M. P.; Nhien N. T.; Imwong M.; Bethell D.; Se Y.; Lon C.; Tyner S. D.; Saunders D. L.; Ariey F.; Mercereau-Puijalon O.; Menard D.; Newton P. N.; Khanthavong M.; Hongvanthong B.; Starzengruber P.; Fuehrer H. P.; Swoboda P.; Khan W. A.; Phyo A. P.; Nyunt M. M.; Nyunt M. H.; Brown T. S.; Adams M.; Pepin C. S.; Bailey J.; Tan J. C.; Ferdig M. T.; Clark T. G.; Miotto O.; MacInnis B.; Kwiatkowski D. P.; White N. J.; Ringwald P.; Plowe C. V. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J. Infect. Dis. 2015, 211, 670–679. 10.1093/infdis/jiu491. - DOI - PMC - PubMed
-
- Baragana B.; Hallyburton I.; Lee M. C. S.; Norcross N. R.; Grimaldi R.; Otto T. D.; Proto W. R.; Blagborough A. M.; Meister S.; Wirjanata G.; Ruecker A.; Upton L. M.; Abraham T. S.; Almeida M. J.; Pradhan A.; Porzelle A.; Martinez M. S.; Bolscher J. M.; Woodland A.; Norval S.; Zuccotto F.; Thomas J.; Simeons F.; Stojanovski L.; Osuna-Cabello M.; Brock P. M.; Churcher T. S.; Sala K. A.; Zakutansky S. E.; Jimenez-Diaz M. B.; Sanz L. M.; Riley J.; Basak R.; Campbell M.; Avery V. M.; Sauerwein R. W.; Dechering K. J.; Noviyanti R.; Campo B.; Frearson J. A.; Angulo-Barturen I.; Ferrer-Bazaga S.; Gamo F. J.; Wyatt P. G.; Leroy D.; Siegl P.; Delves M. J.; Kyle D. E.; Wittlin S.; Marfurt J.; Price R. N.; Sinden R. E.; Winzeler E. A.; Charman S. A.; Bebrevska L.; Gray D. W.; Campbell S.; Fairlamb A. H.; Willis P. A.; Rayner J. C.; Fidock D. A.; Read K. D.; Gilbert I. H. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 2015, 522, 315–320. 10.1038/nature14451. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

