Transplantation of Airway Epithelial Stem/Progenitor Cells: A Future for Cell-Based Therapy
- PMID: 27632244
- PMCID: PMC5248965
- DOI: 10.1165/rcmb.2016-0181MA
Transplantation of Airway Epithelial Stem/Progenitor Cells: A Future for Cell-Based Therapy
Abstract
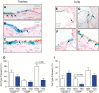
Cell therapy has the potential to cure disease through replacement of malfunctioning cells. Although the tissue stem cell (TSC) is thought to be the optimal therapeutic cell, transplantation of TSC/progenitor cell mixtures has saved lives. We previously purified the mouse tracheobronchial epithelial TSCs and reported that in vitro amplification generated numerous TSCs. However, these cultures also contained TSC-derived progenitor cells and TSC repurification by flow cytometry compromised TSC self-renewal. These limitations prompted us to determine if a TSC/progenitor cell mixture would repopulate the injured airway epithelium. We developed a cell transplantation protocol and demonstrate that transplanted mouse and human tracheobronchial epithelial TSC/progenitor cell mixtures are 20-25% of airway epithelial cells, actively contribute to epithelial repair, and persist for at least 43 days. At 2 weeks after transplantation, TSCs/progenitor cells differentiated into the three major epithelial cell types: basal, secretory, and ciliated. We conclude that cell therapy that uses adult tracheobronchial TSCs/progenitor cells is an effective therapeutic option.
Keywords: airway epithelium; cell therapy; progenitor cell; tissue stem cell.
Figures
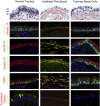
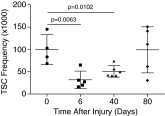
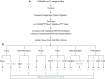
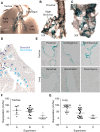

References
-
- Morrison SJ. Stem cell potential: can anything make anything? Curr Biol. 2001;11:R7–R9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

