The Ultrastructural Signature of Human Embryonic Stem Cells
- PMID: 27632380
- PMCID: PMC5558502
- DOI: 10.1002/jcb.25736
The Ultrastructural Signature of Human Embryonic Stem Cells
Abstract
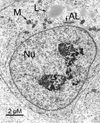
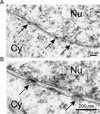
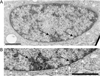
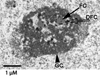
The epigenetics and molecular biology of human embryonic stem cells (hES cells) have received much more attention than their architecture. We present a more complete look at hES cells by electron microscopy, with a special emphasis on the architecture of the nucleus. We propose that there is an ultrastructural signature of pluripotent human cells. hES cell nuclei lack heterochromatin, including the peripheral heterochromatin, that is common in most somatic cell types. The absence of peripheral heterochromatin may be related to the absence of lamins A and C, proteins important for linking chromatin to the nuclear lamina and envelope. Lamins A and C expression and the development of peripheral heterochromatin were early steps in the development of embryoid bodies. While hES cell nuclei had abundant nuclear pores, they also had an abundance of nuclear pores in the cytoplasm in the form of annulate lamellae. These were not a residue of annulate lamellae from germ cells or the early embryos from which hES cells were derived. Subnuclear structures including nucleoli, interchromatin granule clusters, and Cajal bodies were observed in the nuclear interior. The architectural organization of human ES cell nuclei has important implications for cell structure-gene expression relationships and for the maintenance of pluripotency. J. Cell. Biochem. 118: 764-774, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: ANNULATE LAMELLAE; CHROMATIN ORGANIZATION; ELECTRON MICROSCOPY; HUMAN EMBRYONIC STEM CELLS; NUCLEAR STRUCTURE; ULTRASTRUCTURE.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest: The authors have no conflicts to declare.
Figures






References
-
- Baricheva EA, Berrios M, Bogachev SS, Borisevich IV, Lapik ER, Sharakhov IV, Stuurman N, Fisher PA. DNA from Drosophila melanogaster beta-heterochromatin binds specifically to nuclear lamins in vitro and the nuclear envelope in situ. Gene. 1996;171:171–176. - PubMed
-
- Bernhard W. A new procedure for electron microscopical cytology. J. Ultrastruct. Res. 1969;27:250–265. - PubMed
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

