IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa
- PMID: 27632536
- PMCID: PMC5025078
- DOI: 10.1371/journal.ppat.1005882
IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa
Abstract
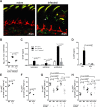
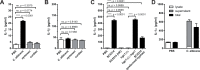
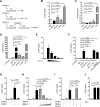
Mucosal infections with Candida albicans belong to the most frequent forms of fungal diseases. Host protection is conferred by cellular immunity; however, the induction of antifungal immunity is not well understood. Using a mouse model of oropharyngeal candidiasis (OPC) we show that interleukin-1 receptor (IL-1R) signaling is critical for fungal control at the onset of infection through its impact on neutrophils at two levels. We demonstrate that both the recruitment of circulating neutrophils to the site of infection and the mobilization of newly generated neutrophils from the bone marrow depended on IL-1R. Consistently, IL-1R-deficient mice displayed impaired chemokine production at the site of infection and defective secretion of granulocyte colony-stimulating factor (G-CSF) in the circulation in response to C. albicans. Strikingly, endothelial cells were identified as the primary cellular source of G-CSF during OPC, which responded to IL-1α that was released from keratinocytes in the infected tissue. The IL-1-dependent crosstalk between two different cellular subsets of the nonhematopoietic compartment was confirmed in vitro using a novel murine tongue-derived keratinocyte cell line and an established endothelial cell line. These data establish a new link between IL-1 and granulopoiesis in the context of fungal infection. Together, we identified two complementary mechanisms coordinating the neutrophil response in the oral mucosa, which is critical for preventing fungal growth and dissemination, and thus protects the host from disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
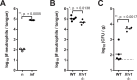




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

