Role of a Conserved Tyrosine Residue in the FMN-Heme Interdomain Electron Transfer in Inducible Nitric Oxide Synthase
- PMID: 27633182
- PMCID: PMC5526657
- DOI: 10.1021/acs.jpca.6b08207
Role of a Conserved Tyrosine Residue in the FMN-Heme Interdomain Electron Transfer in Inducible Nitric Oxide Synthase
Abstract
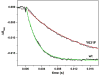
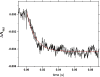
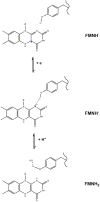
The interdomain electron transfer (IET) between the flavin mononucleotide (FMN) and heme domains is essential in the biosynthesis of nitric oxide (NO) by the NO synthase (NOS) enzymes. A conserved tyrosine residue in the FMN domain (Y631 in human inducible NOS) was proposed to be a key part of the electron transfer pathway in the FMN/heme docked complex model. In the present study, the FMN-heme IET kinetics in the Y631F mutant and wild type of a bidomain oxygenase/FMN construct of human inducible NOS were determined by laser flash photolysis. The rate constant of the Y631F mutant is significantly decreased by ∼75% (compared to the wild type), showing that the tyrosine residue indeed facilitates the FMN-heme IET through the protein medium. The IET rate constant of the wild type protein decreases from 345 to 242 s-1 on going from H2O to 95% D2O, giving a solvent kinetic isotope effect of 1.4. In contrast, no deuterium isotope effect was observed for the Tyr-to-Phe mutant. Moreover, an appreciable change in the wild type iNOS IET rate constant value was observed upon changing pH. These results indicate that the FMN-heme IET is proton coupled, in which the conserved tyrosine residue may play an important role.
Figures



References
-
- Schmidt H, Walter U. NO at Work. Cell. 1994;78:919–925. - PubMed
-
- Lundberg JO, Weitzberg E, Gladwin MT. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat Rev Drug Discov. 2008;7:156–167. - PubMed
-
- Lancaster JR, Xie KP. Tumors Face NO Problems? Cancer Res. 2006;66:6459–6462. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

