Integrated stress response of vertebrates is regulated by four eIF2α kinases
- PMID: 27633668
- PMCID: PMC5025754
- DOI: 10.1038/srep32886
Integrated stress response of vertebrates is regulated by four eIF2α kinases
Abstract
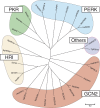
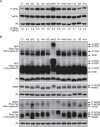
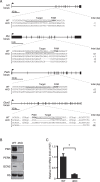
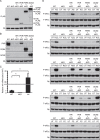
The integrated stress response (ISR) is a cytoprotective pathway initiated upon phosphorylation of the eukaryotic translation initiation factor 2 (eIF2α) residue designated serine-51, which is critical for translational control in response to various stress conditions. Four eIF2α kinases, namely heme-regulated inhibitor (HRI), protein kinase R (PKR), PKR-like endoplasmic reticulum kinase, (PERK) and general control non-depressible 2 (GCN2), have been identified thus far, and they are known to be activated by heme depletion, viral infection, endoplasmic reticulum stress, and amino acid starvation, respectively. Because eIF2α is phosphorylated under various stress conditions, the existence of an additional eIF2α kinase has been suggested. To validate the existence of the unidentified eIF2α kinase, we constructed an eIF2α kinase quadruple knockout cells (4KO cells) in which the four known eIF2α kinase genes were deleted using the CRISPR/Cas9-mediated genome editing. Phosphorylation of eIF2α was completely abolished in the 4KO cells by various stress stimulations. Our data suggests that the four known eIF2α kinases are sufficient for ISR and that there are no additional eIF2α kinases in vertebrates.
Figures

References
-
- Harding H. P. et al.. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11, 619–633 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

