Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols
- PMID: 27633763
- PMCID: PMC5503843
- DOI: 10.1136/tobaccocontrol-2016-053224
Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols
Abstract
Objective: The aim of this study was to evaluate the distribution, concentration and toxicity of cinnamaldehyde in electronic cigarette (e-cigarette) refill fluids and aerosols.
Methods: The distribution and concentration of cinnamaldehyde were determined in 39 e-cigarette refill fluids plus 6 duplicates using gas chromatography and mass spectrometry (GC/MS). A cinnamaldehyde toxicity profile was established for embryonic and adult cells using a live cell imaging assay, immunocytochemistry, the comet assay and a recovery assay.
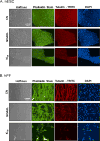
Results: Twenty of the 39 refill fluids contained cinnamaldehyde at concentrations that are cytotoxic to human embryonic and lung cells in the MTT assay. Cinnamon Ceylon aerosol produced in a cartomizer-style e-cigarette was cytotoxic. Cinnamon Ceylon aerosols and refill fluid aerosols (80% propylene glycol or cinnamaldehyde/propylene glycol) made using a tank/boxmod e-cigarette were more cytotoxic at 5 V than 3 V. Using GC/MS, aerosols produced at 5 V contained 10 additional peaks not present in aerosol generated at 3 V. One of these, 2,3-butandione (diacetyl), was confirmed with an authentic standard. Cinnamaldehyde depolymerised microtubules in human pulmonary fibroblasts. At concentrations that produced no effect in the MTT assay, cinnamaldehyde decreased growth, attachment and spreading; altered cell morphology and motility; increased DNA strand breaks; and increased cell death. At the MTT IC50 concentration, lung cells were unable to recover from cinnamaldehyde after 2 hours of treatment, whereas embryonic cells recovered after 8 hours.
Conclusions: Cinnamaldehyde-containing refill fluids and aerosols are cytotoxic, genotoxic and low concentrations adversely affect cell processes and survival. These data indicate that cinnamaldehyde in e-cigarette refill fluids/aerosols may impair homeostasis in the respiratory system.
Keywords: Electronic nicotine delivery devices; Global health; Toxicology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Figures



References
-
- Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2011;20:47–52. - PubMed
-
- Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res. 2010;12:905–12. - PubMed
-
- Hutzler C, Paschke M, Kruschinski S, et al. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88:1295–308. - PubMed
-
- Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, et al. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol. 2015;39:262–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
