Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS)
- PMID: 27633767
- PMCID: PMC5784427
- DOI: 10.1136/tobaccocontrol-2016-053205
Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS)
Abstract
Background: E-cigarettes or electronic nicotine delivery systems (ENDS) are designed to deliver nicotine-containing aerosol via inhalation. Little is known about the health effects of flavoured ENDS aerosol when inhaled.
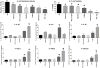
Methods: Aerosol from ENDS was generated using a smoking machine. Various types of ENDS devices or a tank system prefilled with liquids of different flavours, nicotine carrier, variable nicotine concentrations and with modified battery output voltage were tested. A convenience sample of commercial fluids with flavour names of tobacco, piña colada, menthol, coffee and strawberry were used. Flavouring chemicals were identified using gas chromatography/mass spectrometry. H292 human bronchial epithelial cells were directly exposed to 55 puffs of freshly generated ENDS aerosol, tobacco smoke or air (controls) using an air-liquid interface system and the Health Canada intense smoking protocol. The following in vitro toxicological effects were assessed: (1) cell viability, (2) metabolic activity and (3) release of inflammatory mediators (cytokines).
Results: Exposure to ENDS aerosol resulted in decreased metabolic activity and cell viability and increased release of interleukin (IL)-1β, IL-6, IL-10, CXCL1, CXCL2 and CXCL10 compared to air controls. Cell viability and metabolic activity were more adversely affected by conventional cigarettes than most tested ENDS products. Product type, battery output voltage and flavours significantly affected toxicity of ENDS aerosol, with a strawberry-flavoured product being the most cytotoxic.
Conclusions: Our data suggest that characteristics of ENDS products, including flavours, may induce inhalation toxicity. Therefore, ENDS users should use the products with caution until more comprehensive studies are performed.
Keywords: Electronic nicotine delivery devices; Harm Reduction; Toxicology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
MLG reports grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking cessation drugs. Other authors declare no conflict of interest.
References
-
- McGill N. Research on e-cigarettes examining health effects: Regulations due. The Nation’s Health. 2013;43:1–10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical