The efficacy of thymosin α1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials
- PMID: 27633969
- PMCID: PMC5025565
- DOI: 10.1186/s12879-016-1823-5
The efficacy of thymosin α1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials
Abstract
Background: Thymosin α1 (Tα1) as immunomodulatory treatment is supposed to be beneficial for the sepsis patients by regulating T cell subsets and inflammatory mediators. However, limited by the small sample size and the poor study design, the persuasive power of the single clinical studies is weak. This meta-analysis aimed to investigate the impact of Tα1 on the sepsis patients.
Methods: We searched for the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, CBM, VIP, CNKI, WANFANG, Igaku Chuo Zasshi (ICHUSHI) and Korean literature databases reporting the effects of Tα1 on outcomes in sepsis patients.
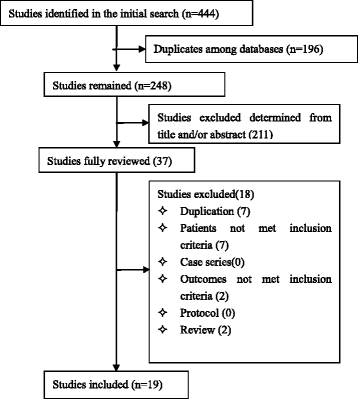
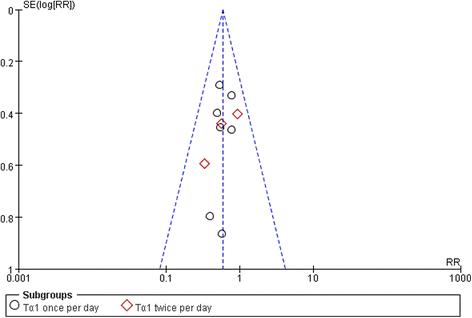
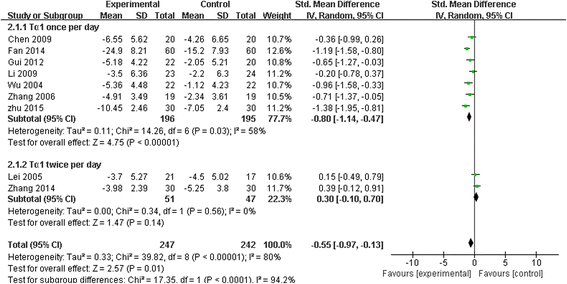
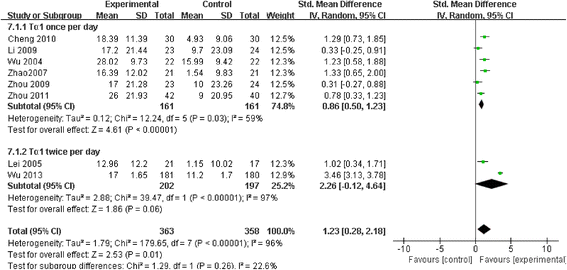
Results: Among 444 related articles, 19 randomized controlled trials (RCTs) met our inclusion criteria. Mortality events were reported in 10 RCTs included 530 patients, and the meta-analysis showed significant decrease in Tα1 group compared with control group (RR 0.59, 95 % CI 0.45 to 0.77, p = 0.0001). The subgroup analysis showed no difference between the two dosages (RR 0.59, 95 % CI 0.43 to 0.81; RR 0.59, 95 % CI 0.35 to 0.98, respectively). In 9 RCTs, with a total of 489 patients, Tα1 administered once per day decrease APACHE II score significantly (SMD -0.80, 95 % CI -1.14 to -0.47, p < 0.0001) while Tα1 twice per day showed no effect (SMD 0.30, 95 % CI-0.10 to 0.70, p = 0.14). However, the length of ICU stay, the incidence of multiple organ failure (MOF) and duration of mechanical ventilation were not significantly affected by Tα1 treatment (SMD -0.52, 95 % CI -1.06 to 0.11, p = 0.06; SMD -0.49, 95 % CI -1.09 to 0.11, p = 0.11; SMD -0.37, 95 % CI -0.90 to 0.17, p = 0.17, respectively). As to the immunological indicators, the level of HLA-DR were increased by Tα1 (SMD 1.23, 95 % CI 0.28 to 2.18, p = 0.01) according to the pooled analysis of 8 studies involving 721 patients. Lymphocyte subsets CD3, CD4 and cytokines IL-6, IL-10 and TNF-α were also beneficially affected by Tα1 treatment.
Conclusions: Tα1 may be beneficial to sepsis patients in reducing mortality and modulating inflammation reactions. However, the quality of evidence supporting the effectiveness is low considering the small sample sizes and inadequate adherence to standardized reporting guidelines for RCTs among the included studies.
Keywords: Immunomodulatory; Sepsis; Systematic review; Thymosin α1.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

