New perspectives on amyotrophic lateral sclerosis: the role of glial cells at the neuromuscular junction
- PMID: 27633977
- PMCID: PMC5285712
- DOI: 10.1113/JP270213
New perspectives on amyotrophic lateral sclerosis: the role of glial cells at the neuromuscular junction
Abstract
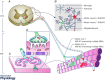
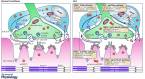
Amyotrophic lateral sclerosis (ALS) is a disease leading to the death of motor neurons (MNs). It is also recognized as a non-cell autonomous disease where glial cells in the CNS are involved in its pathogenesis and progression. However, although denervation of neuromuscular junctions (NMJs) represents an early and major event in ALS, the importance of glial cells at this synapse receives little attention. An interesting possibility is that altered relationships between glial cells and MNs in the spinal cord in ALS may also take place at the NMJ. Perisynaptic Schwann cells (PSCs), which are glial cells at the NMJ, show great morphological and functional adaptability to ensure NMJ stability, maintenance and repair. More specifically, PSCs change their properties according to the state of innervation. Hence, abnormal changes or lack of changes can have detrimental effects on NMJs in ALS. This review will provide an overview of known and hypothesized interactions between MN nerve terminals and PSCs at NMJs during development, aging and ALS-induced denervation. These neuron-PSC interactions may be crucial to the understanding of how degenerative changes begin and progress at NMJs in ALS, and represent a novel therapeutic target.
Keywords: SOD1; denervation; motor unit; non-cell autonomy; perisynaptic Schwann cells; re-innervation; remodeling; synaptic transmission.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
References
-
- Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SS, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, Clare AJ, Badders NM, Bilican B, Chaum E, Chandran S, Shaw CE, Eggan KC, Maniatis T & Taylor JP (2014). Axonal transport of TDP‐43 mRNA granules is impaired by ALS‐causing mutations. Neuron 81, 536–543. - PMC - PubMed
-
- Armstrong GA & Drapeau P (2013. b). Loss and gain of FUS function impair neuromuscular synaptic transmission in a genetic model of ALS. Hum Mol Genet 22, 4282–4292. - PubMed
-
- Atkin JD, Scott RL, West JM, Lopes E, Quah AK & Cheema SS (2005). Properties of slow‐ and fast‐twitch muscle fibres in a mouse model of amyotrophic lateral sclerosis. Neuromuscul Disord 15, 377–388. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

