Update on Poly-ADP-ribose polymerase inhibition for ovarian cancer treatment
- PMID: 27634150
- PMCID: PMC5024442
- DOI: 10.1186/s12967-016-1027-1
Update on Poly-ADP-ribose polymerase inhibition for ovarian cancer treatment
Abstract
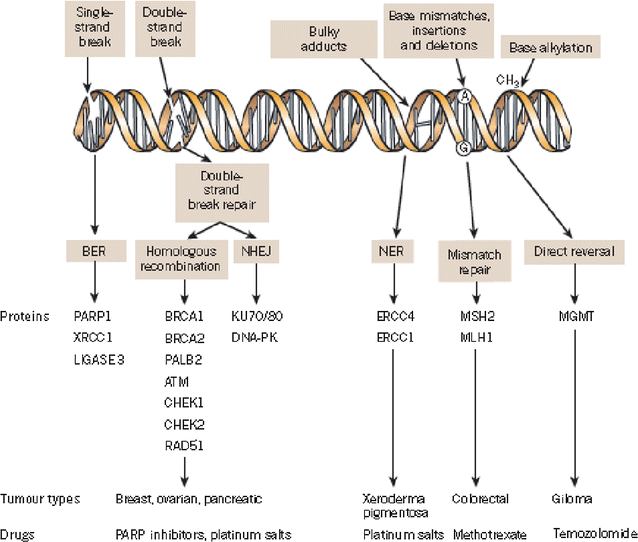
Background: Despite standard treatment for epithelial ovarian cancer (EOC), that involves cytoreductive surgery followed by platinum-based chemotherapy, and initial high response rates to these, up to 80 % of patients experience relapses with a median progression-free survival of 12-18 months. There remains an urgent need for novel targeted therapies to improve clinical outcomes in ovarian cancer. Of the many targeted therapies currently under evaluation, the most promising strategies developed thus far are antiangiogenic agents and Poly(ADP-ribose) polymerase (PARP) inhibitors. Particularly, PARP inhibitors are active in cells that have impaired repair of DNA by the homologous recombination (HR) pathway. Cells with mutated breast related cancer antigens (BRCA) function have HR deficiency, which is also present in a significant proportion of non-BRCA-mutated ovarian cancer ("BRCAness" ovarian cancer). The prevalence of germline BRCA mutations in EOC has historically been estimated to be around 10-15 %. However, recent reports suggest that this may be a gross underestimate, especially in women with high-grade serous ovarian cancer (HGSOC). The emergence of the DNA repair pathway as a rational target in various cancers led to the development of the PARP inhibitors. The concept of tumor-selective synthetic lethality heralded the beginning of an eventful decade, culminating in the approval by regulatory authorities both in Europe as a maintenance therapy and in the United States treatment for advanced recurrent disease of the first oral PARP inhibitor, olaparib, for the treatment of BRCA-mutated ovarian cancer patients. Other PARP inhibitors are clearly effective in this disease and, within the next years, the results of ongoing randomized trials will clarify their respective roles.
Conclusion: This review will discuss the different PARP inhibitors in development and the potential use of this class of agents in the future. Moreover, combination strategies involving PARP inhibitors are likely to receive increasing attention. The utility of PARP inhibitors combined with cytotoxic chemotherapy is of doubtful value, because of enhanced toxicity of this combination; while, more promising strategies include the combination with antiangiogenic agents, or with inhibitors of the P13K/AKT pathway and new generation of immunotherapy.
Keywords: Breast related cancer antigens (BRCA); Epithelial ovarian cancer; Poly-ADP-ribose polymerase inhibitor (PARPi); Target therapy.
Figures
References
-
- Quinn M, Babb B, Brock A, et al. Cancer Trends in England and Wales. Studies on Medical and Population Subjects No. 66. London: The Stationery Office; 2001.
-
- Kusumbe AP, Bapat SA. Ovarian stem cell biology and the emergence of ovarian cancer stem cells. Cancer Stem Cells. 2008;95–110.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

