Cell cycle proliferation decisions: the impact of single cell analyses
- PMID: 27634578
- PMCID: PMC5296213
- DOI: 10.1111/febs.13898
Cell cycle proliferation decisions: the impact of single cell analyses
Abstract
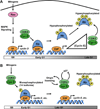
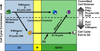
Cell proliferation is a fundamental requirement for organismal development and homeostasis. The mammalian cell division cycle is tightly controlled to ensure complete and precise genome duplication and segregation of replicated chromosomes to daughter cells. The onset of DNA replication marks an irreversible commitment to cell division, and the accumulated efforts of many decades of molecular and cellular studies have probed this cellular decision, commonly called the restriction point. Despite a long-standing conceptual framework of the restriction point for progression through G1 phase into S phase or exit from G1 phase to quiescence (G0), recent technical advances in quantitative single cell analysis of mammalian cells have provided new insights. Significant intercellular heterogeneity revealed by single cell studies and the discovery of discrete subpopulations in proliferating cultures suggests the need for an even more nuanced understanding of cell proliferation decisions. In this review, we describe some of the recent developments in the cell cycle field made possible by quantitative single cell experimental approaches.
Keywords: G0; G1; G2; biosensor; cell cycle; cell division; quiescence; restriction point; review; single cell.
© 2016 Federation of European Biochemical Societies.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

