The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility
- PMID: 27634790
- PMCID: PMC5070751
- DOI: 10.5665/sleep.6226
The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility
Abstract
Study objectives: Obstructive sleep apnea (OSA) results from the interaction of several physiological traits; specifically a compromised upper airway anatomy and muscle function, and two key non-anatomical deficits: elevated loop gain and a low arousal threshold. Although continuous positive airway pressure (CPAP) is an efficacious treatment, it is often poorly tolerated. An alternative approach could involve administering therapies targeting the non-anatomic causes. However, therapies (oxygen or hypnotics) targeting these traits in isolation typically improve, but rarely resolve OSA. Therefore, our aim was to determine how the combination of oxygen and eszopiclone alters the phenotypic traits and OSA severity and to assess the baseline phenotypic characteristics of responders/nonresponders to combination therapy.
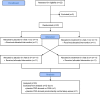
Methods: In a single-blinded randomized crossover study, 20 OSA patients received combination therapy (3 mg eszopiclone and 40% oxygen) versus placebo/sham air, with 1 w between conditions. Under each condition, we assessed the effects on OSA severity (clinical polysomnography) and the phenotypic traits causing OSA using CPAP manipulations (research polysomnography).
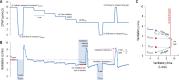
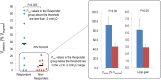
Results: Combination therapy reduced the apnea-hypopnea index (51.9 ± 6.2 vs. 29.5 ± 5.3 events/h; P < 0.001), lowered both the ventilation associated with arousal (5.7 ± 0.3 vs. 5.2 ± 0.3 L/min; P = 0.05) and loop gain (3.3 ± 0.5 vs. 2.2 ± 0.3; P = 0.025). Responders to therapy (apnea-hypopnea index reduced by > 50% to below 15 events/h; n = 9/20) had less severe OSA (P = 0.001), a less collapsible upper airway (P = 0.01) and greater upper airway muscle effectiveness (P = 0.002).
Conclusions: The combination of lowering loop gain and raising the arousal threshold is an effective therapy in patients whose anatomy is not severely compromised. Our work demonstrates that combining therapies that target multiple traits can resolve OSA in selected individuals.
Clinical trial registration: ClinicalTrials.gov, ID: NCT01633827.
Keywords: arousal threshold; loop gain; phenotyping; sleep apnea.
© 2016 Associated Professional Sleep Societies, LLC.
Figures



References
-
- Nieto F, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. - PubMed
-
- Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. - PubMed
-
- Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men [see comments] Lancet. 1990;336:261–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

