The lncRNA HOTAIR impacts on mesenchymal stem cells via triple helix formation
- PMID: 27634931
- PMCID: PMC5159544
- DOI: 10.1093/nar/gkw802
The lncRNA HOTAIR impacts on mesenchymal stem cells via triple helix formation
Abstract
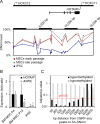
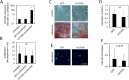
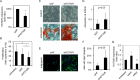
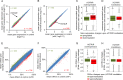
There is a growing perception that long non-coding RNAs (lncRNAs) modulate cellular function. In this study, we analyzed the role of the lncRNA HOTAIR in mesenchymal stem cells (MSCs) with particular focus on senescence-associated changes in gene expression and DNA-methylation (DNAm). HOTAIR binding sites were enriched at genomic regions that become hypermethylated with increasing cell culture passage. Overexpression and knockdown of HOTAIR inhibited or stimulated adipogenic differentiation of MSCs, respectively. Modification of HOTAIR expression evoked only very moderate effects on gene expression, particularly of polycomb group target genes. Furthermore, overexpression and knockdown of HOTAIR resulted in DNAm changes at HOTAIR binding sites. Five potential triple helix forming domains were predicted within the HOTAIR sequence based on reverse Hoogsteen hydrogen bonds. Notably, the predicted triple helix target sites for these HOTAIR domains were also enriched in differentially expressed genes and close to DNAm changes upon modulation of HOTAIR Electrophoretic mobility shift assays provided further evidence that HOTAIR domains form RNA-DNA-DNA triplexes with predicted target sites. Our results demonstrate that HOTAIR impacts on differentiation of MSCs and that it is associated with senescence-associated DNAm. Targeting of epigenetic modifiers to relevant loci in the genome may involve triple helix formation with HOTAIR.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
-
- Roobrouck V.D., Ulloa-Montoya F., Verfaillie C.M. Self-renewal and differentiation capacity of young and aged stem cells. Exp. Cell Res. 2008;314:1937–1944. - PubMed
-
- Koch C.M., Wagner W. Epigenetic biomarker to determine replicative senescence of cultured cells. Methods Mol. Biol. 2013;1048:309–321. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

