Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNγ+ Th1/Tregs
- PMID: 27635087
- PMCID: PMC5242312
- DOI: 10.1161/CIRCRESAHA.116.309764
Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNγ+ Th1/Tregs
Abstract
Rationale: Forkhead box P3+ T regulatory cells (Tregs) are key players in maintaining immune homeostasis. Evidence suggests that Tregs respond to environmental cues to permit or suppress inflammation. In atherosclerosis, Th1-driven inflammation affects Treg homeostasis, but the mechanisms governing this phenomenon are unclear.
Objective: Here, we address whether atherosclerosis impacts Treg plasticity and functionality in Apoe-/- mice, and what effect Treg plasticity might have on the pathology of atherosclerosis.
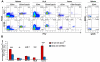
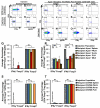
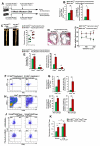
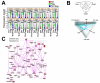
Methods and results: We demonstrate that atherosclerosis promotes Treg plasticity, resulting in the reduction of CXCR3+ Tregs and the accumulation of an intermediate Th1-like interferon (IFN)-γ+CCR5+ Treg subset (Th1/Tregs) within the aorta. Importantly, Th1/Tregs arise in atherosclerosis from bona fide Tregs, rather than from T-effector cells. We show that Th1/Tregs recovered from atherosclerotic mice are dysfunctional in suppression assays. Using an adoptive transfer system and plasticity-prone Mir146a-/- Tregs, we demonstrate that elevated IFNγ+ Mir146a-/- Th1/Tregs are unable to adequately reduce atherosclerosis, arterial Th1, or macrophage content within Apoe-/- mice, in comparison to Mir146a+/+ Tregs. Finally, via single-cell RNA-sequencing and real-time -polymerase chain reaction, we show that Th1/Tregs possess a unique transcriptional phenotype characterized by coexpression of Treg and Th1 lineage genes and a downregulation of Treg-related genes, including Ikzf2, Ikzf4, Tigit, Lilrb4, and Il10. In addition, an ingenuity pathway analysis further implicates IFNγ, IFNα, interleukin-2, interleukin-7, CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), T-cell receptor, and Csnk2b-related pathways in regulating Treg plasticity.
Conclusions: Atherosclerosis drives Treg plasticity, resulting in the accumulation of dysfunctional IFNγ+ Th1/Tregs that may permit further arterial inflammation and atherogenesis.
Keywords: Treg cells; atherosclerosis; cell plasticity; immune system; immunology; inflammation; lymphocytes.
© 2016 American Heart Association, Inc.
Figures



Comment in
-
Foxp3+CD4+ Regulatory T-Cell Subtypes and Atherosclerosis.Circ Res. 2016 Nov 11;119(11):1151-1153. doi: 10.1161/CIRCRESAHA.116.309999. Circ Res. 2016. PMID: 28051776 No abstract available.
References
-
- Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. - PubMed
-
- Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

