mTOR inhibitors in cancer therapy
- PMID: 27635236
- PMCID: PMC5007757
- DOI: 10.12688/f1000research.9207.1
mTOR inhibitors in cancer therapy
Abstract
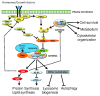
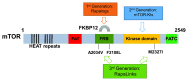
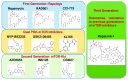
The mammalian target of rapamycin, mTOR, plays key roles in cell growth and proliferation, acting at the catalytic subunit of two protein kinase complexes: mTOR complexes 1 and 2 (mTORC1/2). mTORC1 signaling is switched on by several oncogenic signaling pathways and is accordingly hyperactive in the majority of cancers. Inhibiting mTORC1 signaling has therefore attracted great attention as an anti-cancer therapy. However, progress in using inhibitors of mTOR signaling as therapeutic agents in oncology has been limited by a number of factors, including the fact that the classic mTOR inhibitor, rapamycin, inhibits only some of the effects of mTOR; the existence of several feedback loops; and the crucial importance of mTOR in normal physiology.
Keywords: cancer therapy; mTOR; mTOR inhibitors; rapamycin.
Conflict of interest statement
The authors declare that they have no competing interests. No competing interests were disclosed. No competing interests were disclosed. No competing interests were disclosed.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

