S-Nitrosylation Proteome Profile of Peripheral Blood Mononuclear Cells in Human Heart Failure
- PMID: 27635260
- PMCID: PMC5007369
- DOI: 10.1155/2016/1384523
S-Nitrosylation Proteome Profile of Peripheral Blood Mononuclear Cells in Human Heart Failure
Abstract
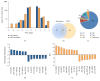
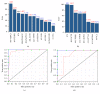
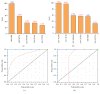
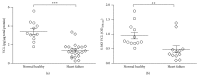
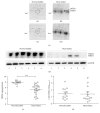
Nitric oxide (NO) protects the heart against ischemic injury; however, NO- and superoxide-dependent S-nitrosylation (S-NO) of cysteines can affect function of target proteins and play a role in disease outcome. We employed 2D-GE with thiol-labeling FL-maleimide dye and MALDI-TOF MS/MS to capture the quantitative changes in abundance and S-NO proteome of HF patients (versus healthy controls, n = 30/group). We identified 93 differentially abundant (59-increased/34-decreased) and 111 S-NO-modified (63-increased/48-decreased) protein spots, respectively, in HF subjects (versus controls, fold-change | ≥1.5|, p ≤ 0.05). Ingenuity pathway analysis of proteome datasets suggested that the pathways involved in phagocytes' migration, free radical production, and cell death were activated and fatty acid metabolism was decreased in HF subjects. Multivariate adaptive regression splines modeling of datasets identified a panel of proteins that will provide >90% prediction success in classifying HF subjects. Proteomic profiling identified ATP-synthase, thrombospondin-1 (THBS1), and vinculin (VCL) as top differentially abundant and S-NO-modified proteins, and these proteins were verified by Western blotting and ELISA in different set of HF subjects. We conclude that differential abundance and S-NO modification of proteins serve as a mechanism in regulating cell viability and free radical production, and THBS1 and VCL evaluation will potentially be useful in the prediction of heart failure.
Figures


References
-
- Alwan A. Global Status Report on Noncommunicable Diseases. Geneva, Switzerland: World Health Organization, WHO Press; 2011. http://www.who.int/nmh/publications/ncd_report_full_en.pdf.
-
- World Health Organization. Chagas Disease: Control and Elimination. Report of the Secretariat. Geneva, Switzerland: UNDP/World Bank/WHO; 2010. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
