Neither vaginal nor buccal administration of 800 μg misoprostol alters mucosal and systemic immune activation or the cervicovaginal microbiome: a pilot study
- PMID: 27636701
- PMCID: PMC5589076
- DOI: 10.1080/13625187.2016.1229765
Neither vaginal nor buccal administration of 800 μg misoprostol alters mucosal and systemic immune activation or the cervicovaginal microbiome: a pilot study
Abstract
Objectives: The aim of the study was to assess the extent to which misoprostol alters mucosal or systemic immune responses following either buccal or vaginal administration.
Methods: This was a prospective, crossover pilot study of 15 healthy, reproductive-age women. Women first received 800 μg misoprostol either via buccal or vaginal administration and were crossed over 1 month later to receive the drug via the other route. Cervicovaginal lavage samples, cervical Cytobrush samples, cervicovaginal swabs, urine and blood were obtained immediately prior to drug administration and the following day. Parameters assessed included urine and cervicovaginal misoprostol levels, whole blood cytokine responses (by ELISA) to immune stimulation with lipopolysaccharide, peripheral blood and cervical lymphocyte phenotyping by flow cytometry, cervicovaginal antimicrobial peptide measurement by ELISA and vaginal microbial ecology assessment by 16S rRNA sequencing.
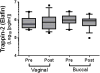
Results: Neither buccal nor vaginal misoprostol significantly altered local or systemic immune and microbiological parameters.
Conclusion: In this pilot study, we did not observe significant alteration of mucosal or systemic immunology or vaginal microbial ecology 1 day after drug administration following either the buccal or vaginal route.
Keywords: Abortion; microbial ecology; mucosal immunology; prostaglandins.
Figures
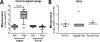
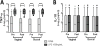
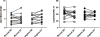
Similar articles
-
Misoprostol administered by epithelial routes: Drug absorption and uterine response.Obstet Gynecol. 2006 Sep;108(3 Pt 1):582-90. doi: 10.1097/01.AOG.0000230398.32794.9d. Obstet Gynecol. 2006. PMID: 16946218 Clinical Trial.
-
Randomized trial of mifepristone and buccal or vaginal misoprostol for abortion through 56 days of last menstrual period.Contraception. 2005 Nov;72(5):328-32. doi: 10.1016/j.contraception.2005.05.017. Epub 2005 Aug 9. Contraception. 2005. PMID: 16246656 Clinical Trial.
-
Comparing vaginal and buccal misoprostol when used after methotrexate for early abortion.Contraception. 2004 Dec;70(6):463-6. doi: 10.1016/j.contraception.2004.05.003. Contraception. 2004. PMID: 15541407 Clinical Trial.
-
[Misoprostol: off-label use in the first trimester of pregnancy (spontaneous abortion, and voluntary medical termination of pregnancy)].J Gynecol Obstet Biol Reprod (Paris). 2014 Feb;43(2):123-45. doi: 10.1016/j.jgyn.2013.11.007. Epub 2014 Jan 13. J Gynecol Obstet Biol Reprod (Paris). 2014. PMID: 24433988 Review. French.
-
Overview and expert assessment of off-label use of misoprostol in obstetrics and gynaecology: review and report by the Collège national des gynécologues obstétriciens français.Eur J Obstet Gynecol Reprod Biol. 2015 Apr;187:80-4. doi: 10.1016/j.ejogrb.2015.01.018. Epub 2015 Jan 31. Eur J Obstet Gynecol Reprod Biol. 2015. PMID: 25701235 Review.
Cited by
-
MAIT Cells Balance the Requirements for Immune Tolerance and Anti-Microbial Defense During Pregnancy.Front Immunol. 2021 Aug 9;12:718168. doi: 10.3389/fimmu.2021.718168. eCollection 2021. Front Immunol. 2021. PMID: 34497611 Free PMC article.
References
-
- Sheldon WR, Blum J, Durocher J, Winikoff B. Misoprostol for the prevention and treatment of postpartum hemorrhage. Expert Opin Investig Drugs. 2012;21:235–50. - PubMed
-
- Chong E, Tsereteli T, Nguyen NN, Winikoff B. A randomized controlled trial of different buccal misoprostol doses in mifepristone medical abortion. Contraception. 2012;86:251–6. - PubMed
-
- Iyengar S, Contreras PC, Mick SJ, et al. Immune modifying effects of misoprostol and natural prostaglandins. Br J Rheumatol. 1991;30(Suppl. 2):71–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
