Imaging Evaluation of Acute Traumatic Brain Injury
- PMID: 27637393
- PMCID: PMC5027071
- DOI: 10.1016/j.nec.2016.05.011
Imaging Evaluation of Acute Traumatic Brain Injury
Abstract
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide. Imaging plays an important role in the evaluation, diagnosis, and triage of patients with TBI. Recent studies suggest that it also helps predict patient outcomes. TBI consists of multiple pathoanatomic entities. This article reviews the current state of TBI imaging including its indications, benefits and limitations of the modalities, imaging protocols, and imaging findings for each of these pathoanatomic entities. Also briefly surveyed are advanced imaging techniques, which include several promising areas of TBI research.
Keywords: CT; Imaging; MRI; TBI; Traumatic brain injury.
Copyright © 2016 Elsevier Inc. All rights reserved.
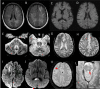
Figures




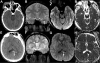

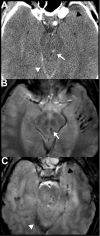
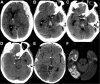

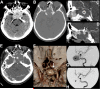
References
-
- Faul MD, Xu L, Wald MM, et al. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta (GA): 2010.
-
- Menon DK, Schwab K, Wright DW, et al. Position Statement: Definition of Traumatic Brain Injury. Archives of physical medicine and rehabilitation. 91(11):1637–1640. - PubMed
-
- Cornelius C, Crupi R, Calabrese V, et al. Traumatic brain injury: oxidative stress and neuroprotection. Antioxidants & redox signaling. 2013;19(8):836–853. - PubMed
-
- Hawryluk GWJ, Manley GT. Chapter 2 - Classification of traumatic brain injury: past, present, and future. In: Jordan G, Andres MS, editors. Handbook of Clinical Neurology. Vol. 127. Elsevier; 2015. pp. 15–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

