A Cluster-Randomized Controlled Trial Evaluating the Effectiveness and Cost-Effectiveness of Tobacco Cessation on Prescription in Swedish Primary Health Care: A Protocol of the Motivation 2 Quit (M2Q) Study
- PMID: 27637517
- PMCID: PMC5045524
- DOI: 10.2196/resprot.6180
A Cluster-Randomized Controlled Trial Evaluating the Effectiveness and Cost-Effectiveness of Tobacco Cessation on Prescription in Swedish Primary Health Care: A Protocol of the Motivation 2 Quit (M2Q) Study
Abstract
Background: In Sweden, the prevalence of tobacco use is disproportionately high among socioeconomically disadvantaged groups. Previous research and clinical experience suggest that prescribed lifestyle interventions in the primary health care (PHC) setting such as Physical Activity on Prescription are effective in changing behavior. However, there is a lack of evidence for if and how such a prescription approach could be effectively transferred into the tobacco cessation context.
Objective: The aim of this trial is to evaluate the effectiveness and cost-effectiveness of Tobacco Cessation on Prescription (TCP) compared to current practice for tobacco cessation targeting socioeconomically disadvantaged groups in the PHC setting in Sweden.
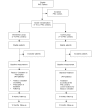
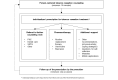
Methods: The design is a pragmatic cluster-randomized controlled trial. The sample will consist of 928 daily tobacco users with Swedish social security numbers and permanent resident permits, recruited from 14-20 PHC centers located in socioeconomically disadvantaged areas in Stockholm County. The primary outcome will be measured in self-reported 7-day abstinence at 6 and 12 months after the intervention. The secondary outcomes will be measured in daily tobacco consumption, number of quit attempts, and health-related quality of life at 6 and 12 months after the intervention. Data will be collected through questionnaires and review of electronic medical records. Cost-effectiveness will be estimated through decision analytic modeling and measured by the incremental cost per quality-adjusted life year.
Results: In the first set of PHC centers participating in the study, eight centers have been included. Recruitment of individual study participants is currently ongoing. Inclusion of a second set of PHC centers is ongoing with expected study start in September 2016.
Conclusions: If TCP is found effective and cost-effective compared to standard treatment, the method could be implemented to facilitate tobacco cessation for socioeconomically disadvantaged groups in the PHC setting in Sweden.
Trial registration: International Standard Randomized Controlled Trial Number (ISRCTN): 11498135; http://www.isrctn.com/ISRCTN11498135 (Archived by WebCite at http://www.webcitation.org/6kTu6giYQ).
Keywords: Sweden; cost-effectiveness analysis; pragmatic clinical trial; primary health care; randomized controlled trial; tobacco use cessation; vulnerable populations.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- National Board of Health and Welfare . [National guidelines for disease prevention methods 2011. Tobacco use, alcohol consumption, physical inactivity and unhealthy dietary habits. Support for control and management] Västerås: Edita Västra Aros; 2011.
-
- National Board of Health and Welfare [Register data on the harmful effects of tobacco use] 2014. [2016-08-02]. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19371/201... .
-
- Agardh E, Boman U, Allebeck P. [Alcohol, drugs and tobacco smoking causes much of the burden of disease--Trends in Sweden 1990-2010 mapped based DALY method] Lakartidningen. 2015;112C4TH - PubMed
-
- Lyons RA, Lo SV, Littlepage B. Perception of Health amongst ever-smokers and never-smokers: a comparison using the SF-36 Health Survey Questionnaire. Tobacco Control. 1994 Sep 01;3(3):213–215. doi: 10.1136/tc.3.3.213. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources