Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy
- PMID: 27637745
- PMCID: PMC8890451
- DOI: 10.1093/annonc/mdw417
Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy
Abstract
Background: The effect of immunologic and targeted agents on intracranial response rates in patients with melanoma brain metastases (MBMs) is not yet clearly understood. This report analyzes outcomes of intact MBMs treated with single-session stereotactic radiosurgery (SRS) and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors(i), BRAFi, or conventional chemotherapy.
Patients and methods: Patients were included if MBMs were treated with single-session SRS within 3 months of receiving systemic therapy. The primary end point of this study was distant MBM control. Secondary end points were local MBM control defined as a >20% volume increase on follow-up MRI, systemic progression-free survival, overall survival (OS) from both SRS and cranial metastases diagnosis, and neurotoxicity. Images were reviewed alongside two neuro-radiologists at our institution.
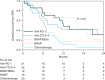
Results: Ninety-six patients were treated to 314 MBMs over 119 SRS treatment sessions between January 2007 and August 2015. No significant differences were noted in age (P = 0.27), gender (P = 0.85), treated gross tumor volume (P = 0.26), or the diagnosis-specific graded prognostic assessment (P = 0.51) between the treatment cohorts. Twelve-month Kaplan-Meier (KM) distant MBM control rates were 38%, 21%, 20%, 8%, and 5% (P = 0.008) for SRS with anti-PD-1 therapies, anti-CTLA-4 therapy, BRAF/MEKi, BRAFi, and conventional chemotherapy, respectively. No significant differences were noted in the KM local MBM control rates among treatment groups (P = 0.25). Treatment with anti-PD-1 therapy, anti-CTLA-4 therapy, or BRAF/MEKi significantly improved OS on both univariate and multivariate analyses when compared with conventional chemotherapy.
Conclusion: In our institutional analysis of patients treated with SRS and various systemic immunologic and targeted melanoma agents, significant differences in distant MBM control and OS are noted. Prospective evaluation of the potential synergistic effect between these agents and SRS is warranted.
Keywords: anti-PD-1 therapy; brain metastases; melanoma; stereotactic radiation.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Davies MA, Liu P, McIntyre Set al. . Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117: 1687–1696. - PubMed
-
- Sperduto PW, Chao ST, Sneed PKet al. . Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010; 77: 655–661. - PubMed
-
- Weber JS, D'Angelo SP, Minor Det al. . Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. - PubMed
-
- Long GV, Stroyakovskiy D, Gogas Het al. . Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015; 386: 444–451. - PubMed
-
- Long GV, Trefzer U, Davies MAet al. . Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 1087–1095. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials