Primary Cilia and Coordination of Receptor Tyrosine Kinase (RTK) and Transforming Growth Factor β (TGF-β) Signaling
- PMID: 27638178
- PMCID: PMC5453389
- DOI: 10.1101/cshperspect.a028167
Primary Cilia and Coordination of Receptor Tyrosine Kinase (RTK) and Transforming Growth Factor β (TGF-β) Signaling
Abstract
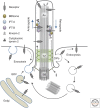
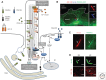
Since the beginning of the millennium, research in primary cilia has revolutionized our way of understanding how cells integrate and organize diverse signaling pathways during vertebrate development and in tissue homeostasis. Primary cilia are unique sensory organelles that detect changes in their extracellular environment and integrate and transmit signaling information to the cell to regulate various cellular, developmental, and physiological processes. Many different signaling pathways have now been shown to rely on primary cilia to function properly, and mutations that lead to ciliary dysfunction are at the root of a pleiotropic group of diseases and syndromic disorders called ciliopathies. In this review, we present an overview of primary cilia-mediated regulation of receptor tyrosine kinase (RTK) and transforming growth factor β (TGF-β) signaling. Further, we discuss how defects in the coordination of these pathways may be linked to ciliopathies.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Absalon S, Blisnick T, Bonhivers M, Kohl L, Cayet N, Toutirais G, Buisson J, Robinson D, Bastin P. 2008. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J Cell Sci 121: 3704–3716. - PubMed
-
- Al JA, Lemaitre AI, Delgehyr N, Faucourt M, Spassky N, Meunier A. 2014. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 516: 104–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
