Persistent Cytomegalovirus Infection in Amniotic Membranes of the Human Placenta
- PMID: 27638253
- PMCID: PMC5222962
- DOI: 10.1016/j.ajpath.2016.07.016
Persistent Cytomegalovirus Infection in Amniotic Membranes of the Human Placenta
Abstract
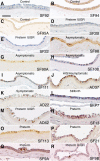
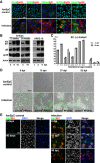
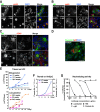
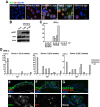
Human cytomegalovirus (HCMV) is the leading viral cause of birth defects, including microcephaly, neurological deficits, hearing impairment, and vision loss. We previously reported that epithelial cells in amniotic membranes of placentas from newborns with intrauterine growth restriction and underlying congenital HCMV infection contain viral proteins in cytoplasmic vesicles. Herein, we immunostained amniotic membranes from 51 placentas from symptomatic and asymptomatic congenital infection with HCMV DNA in amniotic fluid and/or newborn saliva, intrauterine growth restriction, preterm deliveries, and controls. We consistently observed HCMV proteins in amniotic epithelial cells (AmEpCs) from infected placentas, sometimes with aberrant morphology. Primary AmEpCs isolated from mid-gestation placentas infected with pathogenic VR1814 proliferated and released infectious progeny for weeks, producing higher virus titers than late-gestation cells that varied by donor. In contrast to intact virion assembly compartments in differentiated retinal pigment epithelial cells, infected AmEpCs made dispersed multivesicular bodies. Primary AmEpCs and explants of amniochorionic membranes from mid-gestation placentas formed foci of infection, and interferon-β production was prolonged. Infected AmEpCs up-regulated anti-apoptotic proteins survivin and Bcl-xL by mechanisms dependent and independent of the activated STAT3. Amniotic membranes naturally expressed both survivin and Bcl-xL, indicating that fetal membranes could foster persistent viral infection. Our results suggest strengthening innate immune responses and reducing viral functions could suppress HCMV infection in the fetal compartment.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Pass R.F., Stagno S., Myers G.J., Alford C.A. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980;66:758–762. - PubMed
-
- Demmler G.J. Congenital cytomegalovirus infection and disease. Adv Pediatr Infect Dis. 1996;11:135–162. - PubMed
-
- Cannon M.J., Schmid D.S., Hyde T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. - PubMed
-
- Maidji E., Nigro G., Tabata T., McDonagh S., Nozawa N., Shiboski S., Muci S., Anceschi M.M., Aziz N., Adler S.P., Pereira L. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol. 2010;177:1298–1310. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

