Protective effects of alpha phenyl-tert-butyl nitrone and ascorbic acid in human adipose derived mesenchymal stem cells from differently aged donors
- PMID: 27638293
- PMCID: PMC5361667
- DOI: 10.18632/aging.101035
Protective effects of alpha phenyl-tert-butyl nitrone and ascorbic acid in human adipose derived mesenchymal stem cells from differently aged donors
Abstract
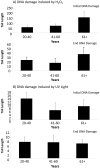
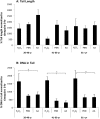
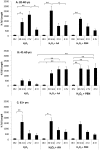
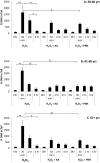
Adipose-derived mesenchymal stem cells (ADSCs) are multipotent stem cells that promote therapeutic effects and are frequently used in autologous applications. Little is known about how ADSCs respond to genotoxic stress and whether or not donor age affects DNA damage and repair. In this study, we used the comet assay to assess DNA damage and repair in human ADSCs derived from young (20-40 years), middle-aged (41-60 years), and older (61+ years) donors following treatment with H2O2 or UV light. Tail lengths in H2O2-treated ADSCs were substantially higher than the tail lengths in UV-treated ADSCs. After 30 minutes of treatment with H2O2, ADSCs preconditioned with alpha phenyl-tert-butyl nitrone (PBN) or ascorbic acid (AA) showed a significant reduction in % tail DNA. The majority of ADSCs treated with PBN or AA displayed low olive tail movements at various timepoints. In general and indicative of DNA repair, % tail length and % tail DNA peaked at 30 minutes and then decreased to near-control levels at the 2 hour and 4 hour timepoints. Differently aged ADSCs displayed comparable levels of DNA damage in the majority of these experiments, suggesting that the age of the donor does not affect the DNA damage response in cultured ADSCs.
Keywords: ADSCs; DNA damage; DNA repair; aging; antioxidants; comet assay.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

