Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants
- PMID: 27638357
- PMCID: PMC5611465
- DOI: 10.1016/S1473-3099(16)30169-4
Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants
Abstract
Background: Identification of mechanisms that limit poliovirus replication is crucial for informing decisions aimed at global polio eradication. Studies of mucosal immunity induced by oral poliovirus (OPV) or inactivated poliovirus (IPV) vaccines and mixed schedules thereof will determine the effectiveness of different vaccine strategies to block virus shedding. We used samples from a clinical trial of different vaccination schedules to measure intestinal immunity as judged by neutralisation of virus and virus-specific IgA in stools.
Methods: In the FIDEC trial, Latin American infants were randomly assigned to nine groups to assess the efficacy of two schedules of bivalent OPV (bOPV) and IPV and challenge with monovalent type 2 OPV, and stools samples were collected. We selected three groups of particular interest-the bOPV control group (serotypes 1 and 3 at 6, 10, and 14 weeks), the trivalent attenuated OPV (tOPV) control group (tOPV at 6, 10, and 14 weeks), and the bOPV-IPV group (bOPV at 6, 10, and 14 weeks plus IPV at 14 weeks). Neutralising activity and poliovirus type-specific IgA were measured in stool after a monovalent OPV type 2 challenge at 18 weeks of age. Mucosal immunity was measured by in-vitro neutralisation of a type 2 polio pseudovirus (PV2). Neutralisation titres and total and poliovirus-type-specific IgG and IgA concentrations in stools were assessed in samples collected before challenge and 2 weeks after challenge from all participants.
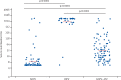
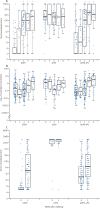
Findings: 210 infants from Guatemala and Dominican Republic were included in this analysis. Of 38 infants tested for mucosal antibody in the tOPV group, two were shedding virus 1 week after challenge, compared with 59 of 85 infants receiving bOPV (p<0·0001) and 53 of 87 infants receiving bOPV-IPV (p<0·0001). Mucosal type 2 neutralisation and type-specific IgA were noted primarily in response to tOPV. An inverse correlation was noted between virus shedding and both serum type 2 neutralisation at challenge (p<0·0001) and mucosal type 2 neutralisation at challenge (p<0·0001).
Interpretation: Mucosal type-2-specific antibodies can be measured in stool and develop in response to receipt of OPV type 2 either in the primary vaccine series or at challenge. These mucosal antibodies influence the amount of virus that is shed in an established infection.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2016 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license. Published by Elsevier Ltd.. All rights reserved.
Figures





Comment in
-
Unravelling mucosal immunity to poliovirus.Lancet Infect Dis. 2016 Dec;16(12):1310-1311. doi: 10.1016/S1473-3099(16)30371-1. Epub 2016 Sep 13. Lancet Infect Dis. 2016. PMID: 27638358 No abstract available.
References
-
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015;10:791–808. - PubMed
-
- Modlin J, Wenger J. Achieving and maintaining polio eradication—new strategies. N Engl J Med. 2014;371:1476–1479. - PubMed
-
- Landaverde JM, Trumbo SP, Danovaro-Holliday MC, Cochi SE, Gandhi R, Ruiz-Matus C. Vaccine-associated paralytic poliomyelitis in the postelimination era in Latin America and the Caribbean, 1992–2011. J Infect Dis. 2014;209:1393–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

