Hepatic transcriptomic alterations for N,N-dimethyl-p-toluidine (DMPT) and p-toluidine after 5-day exposure in rats
- PMID: 27638505
- PMCID: PMC5364093
- DOI: 10.1007/s00204-016-1831-7
Hepatic transcriptomic alterations for N,N-dimethyl-p-toluidine (DMPT) and p-toluidine after 5-day exposure in rats
Abstract
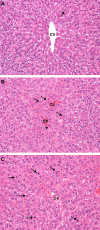
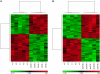
N,N-dimethyl-p-toluidine (DMPT), an accelerant for methyl methacrylate monomers in medical devices, was a liver carcinogen in male and female F344/N rats and B6C3F1 mice in a 2-year oral exposure study. p-Toluidine, a structurally related chemical, was a liver carcinogen in mice but not in rats in an 18-month feed exposure study. In this current study, liver transcriptomic data were used to characterize mechanisms in DMPT and p-toluidine liver toxicity and for conducting benchmark dose (BMD) analysis. Male F344/N rats were exposed orally to DMPT or p-toluidine (0, 1, 6, 20, 60 or 120 mg/kg/day) for 5 days. The liver was examined for lesions and transcriptomic alterations. Both chemicals caused mild hepatic toxicity at 60 and 120 mg/kg and dose-related transcriptomic alterations in the liver. There were 511 liver transcripts differentially expressed for DMPT and 354 for p-toluidine at 120 mg/kg/day (false discovery rate threshold of 5 %). The liver transcriptomic alterations were characteristic of an anti-oxidative damage response (activation of the Nrf2 pathway) and hepatic toxicity. The top cellular processes in gene ontology (GO) categories altered in livers exposed to DMPT or p-toluidine were used for BMD calculations. The lower confidence bound benchmark doses for these chemicals were 2 mg/kg/day for DMPT and 7 mg/kg/day for p-toluidine. These studies show the promise of using 5-day target organ transcriptomic data to identify chemical-induced molecular changes that can serve as markers for preliminary toxicity risk assessment.
Keywords: Liver toxicity; Molecular markers; N,N-dimethyl-p-toluidine; p-Toluidine.
Figures


References
-
- Ahmed MM, Wang T, Luo Y, et al. Aldo-keto reductase-7A protects liver cells and tissues from acetaminophen-induced oxidative stress and hepatotoxicity. Hepatology. 2011;54(4):1322–1332. - PubMed
-
- Auerbach SS, Shah RR, Mav D, et al. Predicting the hepatocarcinogenic potential of alkenylbenzene flavoring agents using toxicogenomics and machine learning. Toxicol Appl Pharmacol. 2010;243(3):300–314. - PubMed
-
- Boutros PC, Yao CQ, Watson JD, et al. Hepatic transcriptomic responses to TCDD in dioxin-sensitive and dioxin-resistant rats during the onset of toxicity. Toxicol Appl Pharmacol. 2011;251(2):119–129. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

