Risk factor SORL1: from genetic association to functional validation in Alzheimer's disease
- PMID: 27638701
- PMCID: PMC5073117
- DOI: 10.1007/s00401-016-1615-4
Risk factor SORL1: from genetic association to functional validation in Alzheimer's disease
Abstract
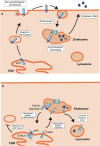
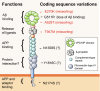
Alzheimer's disease (AD) represents one of the most dramatic threats to healthy aging and devising effective treatments for this devastating condition remains a major challenge in biomedical research. Much has been learned about the molecular concepts that govern proteolytic processing of the amyloid precursor protein to amyloid-β peptides (Aβ), and how accelerated accumulation of neurotoxic Aβ peptides underlies neuronal cell death in rare familial but also common sporadic forms of this disease. Out of a plethora of proposed modulators of amyloidogenic processing, one protein emerged as a key factor in AD pathology, a neuronal sorting receptor termed SORLA. Independent approaches using human genetics, clinical pathology, or exploratory studies in animal models all converge on this receptor that is now considered a central player in AD-related processes by many. This review will provide a comprehensive overview of the evidence implicating SORLA-mediated protein sorting in neurodegenerative processes, and how receptor gene variants in the human population impair functional receptor expression in sporadic but possibly also in autosomal-dominant forms of AD.
Figures



References
-
- Alexopoulos P, Guo LH, Tsolakidou A, Kratzer M, Grimmer T, Westerteicher C, Jiang M, Bujo H, Diehl-Schmid J, Kurz A, et al. Interrelations between CSF soluble AbetaPPbeta, amyloid-beta 1-42, SORL1, and tau levels in Alzheimer’s disease. J Alzheimers Dis. 2012;28:543–552. - PubMed
-
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. - DOI - PMC - PubMed
-
- Andersen OM, Schmidt V, Spoelgen R, Gliemann J, Behlke J, Galatis D, McKinstry WJ, Parker MW, Masters CL, Hyman BT, et al. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006;45:2618–2628. doi: 10.1021/bi052120v. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

