Genomic Epidemiology of Gonococcal Resistance to Extended-Spectrum Cephalosporins, Macrolides, and Fluoroquinolones in the United States, 2000-2013
- PMID: 27638945
- PMCID: PMC5091375
- DOI: 10.1093/infdis/jiw420
Genomic Epidemiology of Gonococcal Resistance to Extended-Spectrum Cephalosporins, Macrolides, and Fluoroquinolones in the United States, 2000-2013
Abstract
Background: Treatment of Neisseria gonorrhoeae infection is empirical and based on population-wide susceptibilities. Increasing antimicrobial resistance underscores the potential importance of rapid diagnostic tests, including sequence-based tests, to guide therapy. However, the usefulness of sequence-based diagnostic tests depends on the prevalence and dynamics of the resistance mechanisms.
Methods: We define the prevalence and dynamics of resistance markers to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in 1102 resistant and susceptible clinical N. gonorrhoeae isolates collected from 2000 to 2013 via the Centers for Disease Control and Prevention's Gonococcal Isolate Surveillance Project.
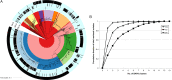
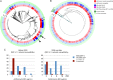
Results: Reduced extended-spectrum cephalosporin susceptibility is predominantly clonal and associated with the mosaic penA XXXIV allele and derivatives (sensitivity 98% for cefixime and 91% for ceftriaxone), but alternative resistance mechanisms have sporadically emerged. Reduced azithromycin susceptibility has arisen through multiple mechanisms and shows limited clonal spread; the basis for resistance in 36% of isolates with reduced azithromycin susceptibility is unclear. Quinolone-resistant N. gonorrhoeae has arisen multiple times, with extensive clonal spread.
Conclusions: Quinolone-resistant N. gonorrhoeae and reduced cefixime susceptibility appear amenable to development of sequence-based diagnostic tests, whereas the undefined mechanisms of resistance to ceftriaxone and azithromycin underscore the importance of phenotypic surveillance. The identification of multidrug-resistant isolates highlights the need for additional measures to respond to the threat of untreatable gonorrhea.
Keywords: Neisseria gonorrhoeae; antibiotic resistance; cephalosporins; fluoroquinolones; genomic epidemiology; gonorrhea; macrolides; molecular diagnostics.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



Comment in
-
Codon 91 Gyrase A Testing Is Necessary and Sufficient to Predict Ciprofloxacin Susceptibility in Neisseria gonorrhoeae.J Infect Dis. 2017 Feb 1;215(3):491. doi: 10.1093/infdis/jiw551. J Infect Dis. 2017. PMID: 28003354 Free PMC article. No abstract available.
-
Reply to Allan-Blitz and Klausner.J Infect Dis. 2017 Feb 1;215(3):491-492. doi: 10.1093/infdis/jiw552. J Infect Dis. 2017. PMID: 28003355 Free PMC article. No abstract available.
References
-
- Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007; 56:332–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

