Nationwide Evaluation of Congenital Hypothyroidism Screening during Neonatal Extracorporeal Membrane Oxygenation
- PMID: 27639769
- PMCID: PMC5296890
- DOI: 10.1159/000448238
Nationwide Evaluation of Congenital Hypothyroidism Screening during Neonatal Extracorporeal Membrane Oxygenation
Abstract
Background: Thyroid hormone concentrations may deviate from normal values during critical illness. This condition is known as nonthyroidal illness syndrome (NTIS), and it can influence the results of screening for congenital hypothyroidism (CH) during neonatal extracorporeal membrane oxygenation (ECMO).
Objectives: To determine the incidence of aberrant CH screening results in ECMO-treated neonates, to identify possible determinants, and to follow up patients with abnormal thyroid hormone concentrations.
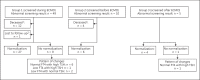
Methods: In this retrospective cohort study, we included 168 ECMO-treated neonates admitted from 2004 to 2014 and screened by protocol and divided them into the following 3 groups: group 1 (screened during ECMO, n = 107), group 2 (screened shortly before ECMO, n = 26), and group 3 (screened shortly after ECMO, n = 35).
Results: CH screening results were aberrant in 67.3% (72/107) of the neonates screened during ECMO, in 73.1% (19/26) of the neonates screened before ECMO, and in 31.4% (11/35) of the neonates screened after ECMO (p < 0.001). Of the neonates with an aberrant screening result, all but 2 (i.e. 98%) had a low thyroxine concentration with a normal thyrotropin concentration at screening, as is seen in NTIS. None was diagnosed with CH. Mortality did not significantly differ between neonates with an aberrant screening result (32.4%) and neonates with a normal screening result (22.7%; p = 0.18). Screening before ECMO (OR 5.92; 95% CI 1.93-18.20), screening during ECMO (OR 4.49; 95% CI 1.98-10.19), and a higher Pediatric Logistic Organ Dysfunction-2 score (OR 1.31; 95% CI 1.04-1.66) were associated with an aberrant screening result.
Conclusions: Aberrant CH screening results were found in most ECMO-treated neonates screened before or during ECMO, which is likely due to NTIS. Follow-up of thyroid hormone concentrations is best started after recovery from critical illness. Our results suggest that thyroxine therapy is not required during ECMO.
© 2016 The Author(s) Published by S. Karger AG, Basel.
Figures
References
-
- Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev. 1997;18:462–475. - PubMed
-
- Kempers MJ, Lanting CI, van Heijst AF, van Trotsenburg AS, Wiedijk BM, de Vijlder JJ, Vulsma T. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin, and thyroxine-binding globulin measurement: potentials and pitfalls. J Clin Endocrinol Metab. 2006;91:3370–3376. - PubMed
-
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med. 1995;333:1688–1694. - PubMed
-
- Marks SD, Haines C, Rebeyka IM, Couch RM. Hypothalamic-pituitary-thyroid axis changes in children after cardiac surgery. J Clin Endocrinol Metab. 2009;94:2781–2786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical