An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease
- PMID: 27639801
- PMCID: PMC5159222
- DOI: 10.1053/j.gastro.2016.08.057
An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease
Abstract
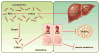
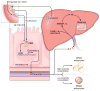
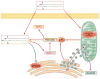
The gut microbiota is associated with metabolic diseases including obesity, insulin resistance, and nonalcoholic fatty liver disease, as shown by correlative studies and by transplant of microbiota from obese humans and mice into germ-free mice. Modification of the microbiota by treatment of high-fat diet (HFD)-fed mice with tempol or antibiotics resulted in decreased adverse metabolic phenotypes. This was owing to lower levels of the genera Lactobacillus and decreased bile salt hydrolase (BSH) activity. The decreased BSH resulted in increased levels of tauro-β-muricholic acid (MCA), a substrate of BSH and a potent farnesoid X receptor (FXR) antagonist. Mice lacking expression of FXR in the intestine were resistant to HFD-induced obesity, insulin resistance, and nonalcoholic fatty liver disease, thus confirming that intestinal FXR is involved in the potentiation of metabolic disease. A potent intestinal FXR antagonist, glycine-β-MCA (Gly-MCA), which is resistant to BSH, was developed, which, when administered to HFD-treated mice, mimics the effect of the altered microbiota on HFD-induced metabolic disease. Gly-MCA had similar effects on genetically obese leptin-deficient mice. The decrease in adverse metabolic phenotype by tempol, antibiotics, and Gly-MCA was caused by decreased serum ceramides. Mice lacking FXR in the intestine also have lower serum ceramide levels, and are resistant to HFD-induced metabolic disease, and this was reversed by injection of C16:0 ceramide. In mouse ileum, because of the presence of endogenous FXR agonists produced in the liver, FXR target genes involved in ceramide synthesis are activated and when Gly-MCA is administered they are repressed, which likely accounts for the decrease in serum ceramides. These studies show that ceramides produced in the ileum under control of FXR influence metabolic diseases.
Keywords: Bile Acids; Ceramides; Farnesoid X Receptor; Metabolic Disease.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interest or conflict of interest.
Figures


References
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. - PubMed
-
- Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–27. - PubMed
-
- Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

