Association of Proton Pump Inhibitors With Reduced Risk of Warfarin-Related Serious Upper Gastrointestinal Bleeding
- PMID: 27639805
- PMCID: PMC5124401
- DOI: 10.1053/j.gastro.2016.08.054
Association of Proton Pump Inhibitors With Reduced Risk of Warfarin-Related Serious Upper Gastrointestinal Bleeding
Abstract
Background & aims: Proton pump inhibitors (PPIs) might reduce the risk of serious warfarin-related upper gastrointestinal bleeding, but the evidence of their efficacy for this indication is limited. A gastroprotective effect of PPIs would be particularly important for patients who take warfarin with antiplatelet drugs or nonselective nonsteroidal anti-inflammatory drugs (NSAIDs), which further increase the risk of gastrointestinal bleeding.
Methods: This retrospective cohort study of patients beginning warfarin treatment in Tennessee Medicaid and the 5% National Medicare Sample identified 97,430 new episodes of warfarin treatment with 75,720 person-years of follow-up. The study end points were hospitalizations for upper gastrointestinal bleeding potentially preventable by PPIs and for bleeding at other sites.
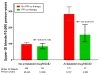
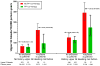
Results: Patients who took warfarin without PPI co-therapy had 119 hospitalizations for upper gastrointestinal bleeding per 10,000 person-years of treatment. The risk decreased by 24% among patients who received PPI co-therapy (adjusted hazard ratio [HR], 0.76; 95% confidence interval [CI], 0.63-0.91). There was no significant reduction in the risk of other gastrointestinal bleeding hospitalizations (HR, 1.07; 95% CI, 0.94-1.22) or non-gastrointestinal bleeding hospitalizations (HR, 0.98; 95% CI, 0.84-1.15) in this group. Among patients concurrently using antiplatelet drugs or NSAIDs, those without PPI co-therapy had 284 upper gastrointestinal bleeding hospitalizations per 10,000 person-years of warfarin treatment. The risk decreased by 45% (HR, 0.55; 95% CI, 0.39-0.77) with PPI co-therapy. PPI co-therapy had no significant protective effect for warfarin patients not using antiplatelet drugs or NSAIDs (HR, 0.86; 95% CI, 0.70-1.06). Findings were similar in both study populations.
Conclusions: In an analysis of patients beginning warfarin treatment in Tennessee Medicaid and the 5% National Medicare Sample, PPI co-therapy was associated with reduced risk of warfarin-related upper gastrointestinal bleeding; the greatest reduction occurred in patients also taking antiplatelet drugs or NSAIDs.
Keywords: Antiplatelet Drugs; Proton-Pump Inhibitor; Warfarin.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest. There are no conflicts of interest for any author to declare.
Figures

References
Reference List
-
- Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–849. - PubMed
-
- Piper JM, Ray WA, Griffin MR, Fought R, Daugherty JR, Mitchel E., Jr Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1990;132:561–571. - PubMed
-
- Ray WA. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–1594. - PubMed
References
-
- Hankey GJ, Eikelboom JW. Dabigatran etexilate: A new oral thrombin inhibitor. Circulation. 2011;123:1436–1450. - PubMed
-
- Wysowsky DK, Nourjah P, Swartz L. Bleeding complications with warfarin use. Arch Intern Med. 2007;167(13):1414–1419. - PubMed
-
- Olsson SB Investigators ESCobotSI. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362(9397):1691–1698. - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

