Laquinimod efficacy in relapsing-remitting multiple sclerosis: how to understand why and if studies disagree
- PMID: 27639853
- PMCID: PMC5027083
- DOI: 10.1186/s12883-016-0702-4
Laquinimod efficacy in relapsing-remitting multiple sclerosis: how to understand why and if studies disagree
Abstract
Background: The results of two randomized phase 3 trials that investigated the use of laquinimod in patients with relapsing-remitting multiple sclerosis were analyzed using a propensity score model.
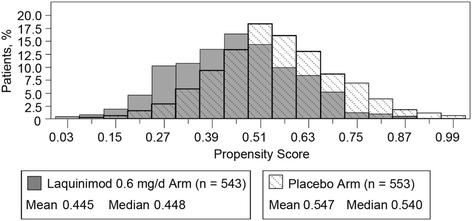
Methods: The propensity score in each study was defined as the probability of an individual patient being assigned to either the laquinimod or placebo study arm. The analysis included two main stages: (1) calculation of a propensity score for each patient, given a broad set of baseline covariates that included second-degree interactions, and (2) incorporation of the propensity score as another covariate into the predefined primary analysis model to test the treatment effect of laquinimod (0.6 mg/d) vs placebo on the annualized relapse rate (ARR).
Results: The BRAVO study showed baseline imbalances for T2 volume and the proportion of patients with gadolinium (Gd)-enhancing lesions, both parameters known to correlate with risk of relapse. Adjustment using the propensity score as a categorical variable showed that the estimated difference in ARR between laquinimod and placebo was 0.078, in favor of laquinimod. In ALLEGRO, the baseline Gd-enhancing lesion mean score was higher for placebo vs laquinimod. When the primary analysis model was adjusted for the propensity score as a categorical variable, the covariate adjusted difference in mean ARR between laquinimod and placebo was 0.084, in favor of laquinimod.
Conclusions: Propensity scores addressing differences in baseline characteristics may be helpful to better understand whether observed treatment effect differences in randomized controlled trials are accurate results or result from inherent differences between patients with multiple sclerosis.
Keywords: Interferon beta-1b; Laquinimod; Propensity score; Relapsing-remitting multiple sclerosis.
Figures
References
-
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. - DOI
-
- Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Di Renzo V. Observational studies: propensity score analysis of non-randomized data. Int MS J. 2009;16:90–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources