Population pharmacokinetics and dosing recommendations for the use of deferiprone in children younger than 6 years
- PMID: 27641003
- PMCID: PMC5306498
- DOI: 10.1111/bcp.13134
Population pharmacokinetics and dosing recommendations for the use of deferiprone in children younger than 6 years
Abstract
Aims: Despite long clinical experience with deferiprone, there is limited information on its pharmacokinetics in children aged <6 years. Here we assess the impact of developmental growth on the pharmacokinetics of deferiprone in this population using a population approach. Based on pharmacokinetic bridging concepts, we also evaluate whether the recommended doses yield appropriate systemic exposure in this group of patients.
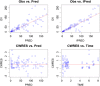
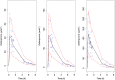
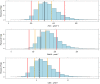
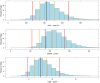
Methods: Data from a study in which 18 paediatric patients were enrolled were available for the purposes of this analysis. Patients were randomised to three deferiprone dose levels (8.3, 16.7 and 33.3 mg kg-1 ). Blood samples were collected according to an optimised sampling scheme in which each patient contributed to a maximum of five samples. A population pharmacokinetic model was developed using NONMEM v.7.2. Model selection criteria were based on graphical and statistical summaries.
Results: A one-compartment model with first-order absorption and first-order elimination best described the pharmacokinetics of deferiprone. Drug disposition parameters were affected by body weight, with both clearance and volume increasing allometrically with size. Simulation scenarios show that comparable systemic exposure (AUC) is achieved in children and adults after similar dose levels in mg kg-1 , with median (5-95th quantiles) AUC values, respectively, of 340.6 (223.2-520.0) μmol l-1 h and 318.5 (200.4-499.0) μmol l-1 h at 75 mg kg-1 day-1 , and 453.7 (297.3-693.0) μmol l-1 h and 424.2 (266.9-664.0) μmol l-1 h at 100 mg kg-1 day-1 given as three times daily (t.i.d.) doses.
Conclusions: Based on the current findings, a dosing regimen of 25 mg kg-1 t.i.d. is recommended in children aged <6 years, with the possibility of titration up to 33.3 mg kg-1 t.i.d.
Keywords: deferiprone; dose rationale; paediatrics; pharmacokinetic bridging; thalassaemia.
© 2016 The British Pharmacological Society.
Figures
References
-
- Gibbons R, Higgs DR, Old JM, Olivieri NF, Swee Lay T, Wood WG. The Thalassaemia Syndromes, Fourth edn. Edinburgh: Blackwell Science, 2001.
-
- Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Complications of beta‐thalassemia major in North America. Blood 2004; 104: 34–39. - PubMed
-
- Borgna‐Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, et al. Survival and complications in thalassemia. Ann N Y Acad Sci 2005; 1054: 40–47. - PubMed
-
- Borgna‐Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, et al. Cardiac morbidity and mortality in deferoxamine‐ or deferiprone‐treated patients with thalassemia major. Blood 2006; 107: 3733–3737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

