CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies
- PMID: 27641304
- PMCID: PMC5135584
- DOI: 10.1016/j.stem.2016.08.006
CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies
Abstract
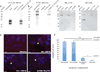
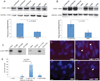
Cell-surface markers for prospective isolation of stem cells from human skeletal muscle have been difficult to identify. Such markers would be powerful tools for studying satellite cell function during homeostasis and in pathogenesis of diseases such as muscular dystrophies. In this study, we show that the tetraspanin KAI/CD82 is an excellent marker for prospectively isolating stem cells from human fetal and adult skeletal muscle. Human CD82+ muscle cells robustly engraft into a mouse model of muscular dystrophy. shRNA knockdown of CD82 in myogenic cells reduces myoblast proliferation, suggesting it is functionally involved in muscle homeostasis. CD82 physically interacts with alpha7beta1 integrin (α7β1-ITG) and with α-sarcoglycan, a member of the Dystrophin-Associated Glycoprotein Complex (DAPC), both of which have been linked to muscular dystrophies. Consistently, CD82 expression is decreased in Duchenne muscular dystrophy patients. Together, these findings suggest that CD82 function may be important for muscle stem cell function in muscular disorders.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Anastasi G, Cutroneo G, Rizzo G, Arco A, Santoro G, Bramanti P, Vitetta AG, Pisani A, Trimarchi F, Favaloro A. Sarcoglycan and integrin localization in normal human skeletal muscle: a confocal laser scanning microscope study. Eur J Histochem. 2004;48:245–252. - PubMed
-
- Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res. 2001;265:212–220. - PubMed
-
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

