Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia
- PMID: 27641501
- PMCID: PMC7360335
- DOI: 10.1016/j.cell.2016.08.057
Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia
Abstract
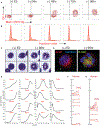
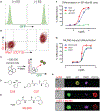
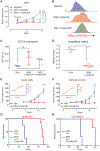
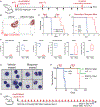
While acute myeloid leukemia (AML) comprises many disparate genetic subtypes, one shared hallmark is the arrest of leukemic myeloblasts at an immature and self-renewing stage of development. Therapies that overcome differentiation arrest represent a powerful treatment strategy. We leveraged the observation that the majority of AML, despite their genetically heterogeneity, share in the expression of HoxA9, a gene normally downregulated during myeloid differentiation. Using a conditional HoxA9 model system, we performed a high-throughput phenotypic screen and defined compounds that overcame differentiation blockade. Target identification led to the unanticipated discovery that inhibition of the enzyme dihydroorotate dehydrogenase (DHODH) enables myeloid differentiation in human and mouse AML models. In vivo, DHODH inhibitors reduced leukemic cell burden, decreased levels of leukemia-initiating cells, and improved survival. These data demonstrate the role of DHODH as a metabolic regulator of differentiation and point to its inhibition as a strategy for overcoming differentiation blockade in AML.
Keywords: HoxA9; acute myeloid leukemia; brequinar; differentiation; dihydroorotate dehydrogenase; high-throughput screen; leukemia-initiating cell; metabolic inhibitor; phenotypic screen.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Lifting the Differentiation Embargo.Cell. 2016 Sep 22;167(1):45-46. doi: 10.1016/j.cell.2016.08.051. Cell. 2016. PMID: 27662083
-
A new target for differentiation therapy in AML.Cell Res. 2017 Jan;27(1):9-10. doi: 10.1038/cr.2016.130. Epub 2016 Nov 11. Cell Res. 2017. PMID: 27834347 Free PMC article.
References
-
- Adamaki M, Lambrou GI, Athanasiadou A, Vlahopoulos S, Papavassiliou AG, and Moschovi M (2015). HOXA9 and MEIS1 gene overexpression in the diagnosis of childhood acute leukemias: significant correlation with relapse and overall survival. Leuk. Res 39, 874–882. - PubMed
-
- Arteaga CL, Brown TD, Kuhn JG, Shen HS, O’Rourke TJ, Beougher K, Brentzel HJ, Von Hoff DD, and Weiss GR (1989). Phase I clinical and pharmacokinetic trial of Brequinar sodium (DuP 785; NSC 368390). Cancer Res 49, 4648–4653. - PubMed
-
- Burris HA 3rd, Raymond E, Awada A, Kuhn JG, O’Rourke TJ, Brentzel J, Lynch W, King SY, Brown TD, and Von Hoff DD (1998). Pharmacokinetic and phase I studies of brequinar (DUP 785; NSC 368390) in combination with cisplatin in patients with advanced malignancies. Invest. New Drugs 16, 19–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

