Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties
- PMID: 27641937
- PMCID: PMC5264143
- DOI: 10.1111/jcmm.12969
Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties
Abstract
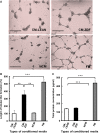
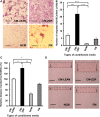
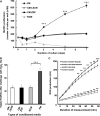
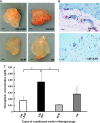
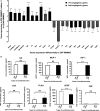
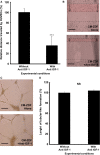
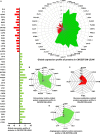
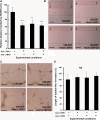
This study aimed at characterizing the impact of type 2 diabetes mellitus (T2DM) on the bone marrow mesenchymal stem cell (BMMSC) secretome and angiogenic properties. BMMSCs from Zucker diabetic fatty rats (ZDF) (a T2DM model) and Zucker LEAN littermates (control) were cultured. The supernatant conditioned media (CM) from BMMSCs of diabetic and control rats were collected and analysed. Compared to results obtained using CM from LEAN-BMMSCs, the bioactive content of ZDF-BMMSC CM (i) differently affects endothelial cell (HUVEC) functions in vitro by inducing increased (3.5-fold; P < 0.01) formation of tubule-like structures and migration of these cells (3-fold; P < 0.001), as well as promotes improved vascular formation in vivo, and (ii) contains different levels of angiogenic factors (e.g. IGF1) and mediators, such as OSTP, CATD, FMOD LTBP1 and LTBP2, which are involved in angiogenesis and/or extracellular matrix composition. Addition of neutralizing antibodies against IGF-1, LTBP1 or LTBP2 in the CM of BMMSCs from diabetic rats decreased its stimulatory effect on HUVEC migration by approximately 60%, 40% or 40%, respectively. These results demonstrate that BMMSCs from T2DM rats have a unique secretome with distinct angiogenic properties and provide new insights into the role of BMMSCs in aberrant angiogenesis in the diabetic milieu.
Keywords: MSCs; angiogenesis; diabetes type 2; endothelial cells; secretome.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
The Fate Status of Stem Cells in Diabetes and its Role in the Occurrence of Diabetic Complications.Front Mol Biosci. 2021 Nov 2;8:745035. doi: 10.3389/fmolb.2021.745035. eCollection 2021. Front Mol Biosci. 2021. PMID: 34796200 Free PMC article. Review.
-
Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells.Cells. 2021 Jan 29;10(2):268. doi: 10.3390/cells10020268. Cells. 2021. PMID: 33572905 Free PMC article.
-
Impact of mesenchymal stem cells' secretome on glioblastoma pathophysiology.J Transl Med. 2017 Oct 2;15(1):200. doi: 10.1186/s12967-017-1303-8. J Transl Med. 2017. PMID: 28969635 Free PMC article.
-
Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis.Mol Med Rep. 2015 Jul;12(1):20-30. doi: 10.3892/mmr.2015.3409. Epub 2015 Mar 4. Mol Med Rep. 2015. PMID: 25739039 Free PMC article.
-
Deconstructing type 2 diabetes.Cell. 1999 Apr 2;97(1):9-12. doi: 10.1016/s0092-8674(00)80709-6. Cell. 1999. PMID: 10199397 Review. No abstract available.
Cited by
-
Vascular Endothelial Cell Biology: An Update.Int J Mol Sci. 2019 Sep 7;20(18):4411. doi: 10.3390/ijms20184411. Int J Mol Sci. 2019. PMID: 31500313 Free PMC article. Review.
-
Impact of Diabetes Mellitus on the Potential of Autologous Stem Cells and Stem Cell-Derived Microvesicles to Repair the Ischemic Heart.Cardiovasc Drugs Ther. 2022 Oct;36(5):933-949. doi: 10.1007/s10557-021-07208-9. Epub 2021 Jul 12. Cardiovasc Drugs Ther. 2022. PMID: 34251593 Review.
-
Therapeutic Effects of Adipose Stem Cells from Diabetic Mice for the Treatment of Type 2 Diabetes.Mol Ther. 2018 Aug 1;26(8):1921-1930. doi: 10.1016/j.ymthe.2018.06.013. Epub 2018 Jun 19. Mol Ther. 2018. PMID: 30005867 Free PMC article.
-
Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus.Int J Nanomedicine. 2017 Sep 26;12:7089-7101. doi: 10.2147/IJN.S124584. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29026303 Free PMC article.
-
The Fate Status of Stem Cells in Diabetes and its Role in the Occurrence of Diabetic Complications.Front Mol Biosci. 2021 Nov 2;8:745035. doi: 10.3389/fmolb.2021.745035. eCollection 2021. Front Mol Biosci. 2021. PMID: 34796200 Free PMC article. Review.
References
-
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003; 23: 117–45. - PubMed
-
- Abu El‐Asrar AM, Nawaz MI, Kangave D, et al Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. 2013; 50: 545–51. - PubMed
-
- Abu El‐Asrar AM, Struyf S, Kangave D, et al Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006; 17: 155–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

