Complement component 3 deficiency prolongs MHC-II disparate skin allograft survival by increasing the CD4(+) CD25(+) regulatory T cells population
- PMID: 27641978
- PMCID: PMC5027598
- DOI: 10.1038/srep33489
Complement component 3 deficiency prolongs MHC-II disparate skin allograft survival by increasing the CD4(+) CD25(+) regulatory T cells population
Abstract
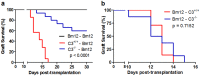
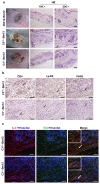
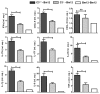
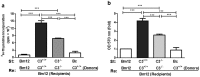
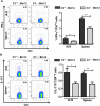
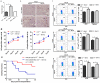
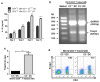
Recent reports suggest that complement system contributes to allograft rejection. However, its underlying mechanism is poorly understood. Herein, we investigate the role of complement component 3 (C3) in a single MHC-II molecule mismatched murine model of allograft rejection using C3 deficient mice (C3(-/-)) as skin graft donors or recipients. Compared with C3(+/+) B6 allografts, C3(-/-) B6 grafts dramatically prolonged survival in MHC-II molecule mismatched H-2(bm12) B6 recipients, indicating that C3 plays a critical role in allograft rejection. Compared with C3(+/+) allografts, both Th17 cell infiltration and Th1/Th17 associated cytokine mRNA levels were clearly reduced in C3(-/-) allografts. Moreover, C3(-/-) allografts caused attenuated Th1/Th17 responses, but increased CD4(+)CD25(+)Foxp3(+) regulatory T (Treg) cell expression markedly in local intragraft and H-2(bm12) recipients. Depletion of Treg cells by anti-CD25 monoclonal antibody (mAb) negated the survival advantages conferred by C3 deficiency. Our results indicate for the first time that C3 deficiency can prolong MHC-II molecule mismatched skin allograft survival, which is further confirmed to be associated with increased CD4(+) CD25(+) Treg cell population expansion and attenuated Th1/Th17 response.
Figures
References
-
- Walport M. J. Complement. First of two parts. The New England journal of medicine 344, 1058–1066 (2001). - PubMed
-
- Kemper C. & Atkinson J. P. T-cell regulation: with complements from innate immunity. Nature reviews. Immunology 7, 9–18 (2007). - PubMed
-
- Carroll M. C. The complement system in regulation of adaptive immunity. Nature immunology 5, 981–986 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

